Abstract
Bacteria have developed the capability to produce structured communities (or cluster of cells) via adherence to surface to form biofilms that facilitate or prolong their survival under extreme environmental condition. Bacterial biomass adheres to inanimate and biotic surfaces in the hospital setting as well as in the environment. In the healthcare system, the biofilm formation on medical devices allows bacteria to sustain as a reservoir and becomes more resistant to antimicrobial agents. However, biofilm formation facilitates pathogens to sabotage the host defenses that are linked to long-term retention within the host cell. Therefore, in this review, we provide some steps leading to the formation of biofilm within the host and on inanimate surfaces, also emphasizing various medically significant pathogens and debate current developments on novel approaches that aimed to prevent biofilm formations and its dispersion to patients.
Keywords
- biofilm
- antibiotic resistant
- distribution
- control
- therapeutic strategy
1. Introduction
Biofilm formation is structured accumulation of fastidious microorganisms attached on inanimate objects or compact surfaces that extensively have been examined in the past decades because they particularly cause infections and more often responsible for chronic infections [1, 2, 3]. They are predominantly problematic due to their antimicrobial resistant properties and their ability to evade host defense mechanisms, which substantially hinders disease treatment in the hospital [1, 2, 3, 4]. Bacterial biofilms are ubiquitous in nature and harbor phenotypic adaptations in the environment with respect to broader perspective [1]. The nature of single cell organisms enables them to adhere to each other and form a “complex structure,” which assists to survive under adverse environmental condition. The biofilm formation occurs from planktonic bacteria due to environmental changes and involves in conjugation gene transfer “multiple regulatory network” from one bacterium to another in response to environmental stress [5, 6, 7, 8, 9]. This type of cell-to-cell adhesion and gene transformation changes the expression of surface molecules, virulence factors, and nutrient utilization that enables their survival under unfavorable environmental condition [8, 10, 11, 12, 13, 14, 15, 16, 17].
Bacteria are cocooned within the biofilm and form extracellular matrix, which represents 90% of the biomass [18]. The matrix as a stabilizing scaffold for the three-dimensional structure is composed of extracellular polymeric substance (EPS) along with extracellular DNS and carbohydrate binding protein [19, 20, 21]. Nutrients are trapped by the resident bacteria in the matrix and water is retained efficiently via H-bond interaction with hydrophilic polysaccharides [18, 22]. The composition of extracellular polymeric substance (EPS) is modified in response to alterations in nutrient availability [23, 24] by certain enzyme secretion of bacteria, thus tailoring biofilm formation to the more specific environment [23, 25]. Therefore, the skeletal components of the extracellular matrix are highly hydrated and provide high tensile strength that enables bacteria to exchange their DNA by conjugation and promote cell-to-cell interaction while defending the biomass from predation, radiation, desiccation, oxidizing molecules, and other dangerous agents [18, 26, 27, 28].
The multifaceted nature of biofilms that allow the bacteria to form a community, i.e., division of labor and express their virulence factors in response to local oxygen and nutrient availability, makes them resistant against different antimicrobial agents [29, 30]. Some studies have shown that there are presence of nondividing metabolically inactive recalcitrant bacteria within the biomass [29, 31], which play very crucial role to cause tolerance against broad-spectrum antimicrobial drugs. The matrix protein inside the host cell protects bacterial biofilm against innate immune defenses, i.e., phagocytosis and opsonization [32]. The spread of other virulence factors inside the host cell and drug resistance marker is due to the cell-to-cell interaction [15]. Thus, biofilm-forming pathogens retained and adhere to the infected surface and cause recalcitrant and chronic infection, i.e., upper respiratory tract infection (particularly,
2. Extracellular formation of biofilm
2.1 Bacterial attachment on surfaces and what does make it adhere to object surface?
Bacterial biofilm growth, subsequent maturation, and aggregation consist of irreversible and reversible stages, which involve various conserved and species-specific aspects. At the first stage, the bacteria are introduced on the surface; a process of at least a part of stochastic that is driven by gravitational forces and Brownian motion, and usually influenced by nearby hydrodynamic forces [39, 40]. Microorganisms encounter with repelling or attractive forces—within the niche that alter depending on ionic strength, pH, nutrient levels, and temperature. Bacterial cell wall composition, along with medium properties, affects direction and velocity toward or away by the contact surface of pathogens [39]. Motile bacteria utilize flagella in order to overcome repulsive and hydrodynamic forces, by having a competitive advantage. The main function of flagella is to provide motility and initial cell attachment to the surface for various pathogens, including
Initially, the attachment is reversible and dynamic during which pathogens can separate and rejoin planktonic biomass if agitated through repulsive forces [48], hydrodynamic forces—detach bacteria off from the surface. Some bacteria attained irreversible attachment in order to maintain a firm grip on the cell surface. Serotypes of other
Furthermore, antigen 43, curli fibers, and type 1 pili have been observed to facilitate attachment and cell-to-cell interaction on inanimate surfaces [57]. Curli fiber also mediates attachment to the extracellular matrix components in eukaryotes such as plasminogen, fibronectin, and laminin [58].
2.2 Maturation of biofilm
Cell-to-cell interaction triggers specific intrinsic responses that cause changes in the gene expression, upregulating factors favorable to sessility especially for those involved in extracellular matrix protein formation [40]. However, relatively very little information is obtained about the matrix constituents with respect to
Mature
2.3 Matrix escape mechanisms
Bacterial mature biofilm provides a suitable living environment to the resident microorganisms for making compact surface adherence community, so as to share products and actively exchange their genetic materials by conjugation. Moreover, as biofilms mature, dispersal becomes a choice. In addition to passive dispersal caused by shear stress, the pathogen develops different ways to recognize environmental changes, which make it to stay within the biofilm. Bacterial biofilm dispersal occurs as a result of various clues such as oxygen fluctuations, modifications in nutrient availability, and increases in toxic products [74]. Biofilm dispersal is induced by the increase of extracellular iron in uropathogenic
Recently, some results reported the factors responsible for such changes such as downregulation of extracellular polymeric substance, reduction of cyclic-di-GMP in bacterial biofilm communities, and upregulation of swarming and swimming motility [25]. Certain type of enzymes (such as alginate lyase) also participates in pathogen detachment from surface especially in
Due to dispersing nature of bacteria, they may have the ability to restart the biofilm formation process after encountering a favorable environmental condition [81]. This is another sophisticated mechanism of dispersal revealed by using
3. Bacterial intracellular biofilms
Gathering evidence have showed that numerous bacterial pathogenic species formerly considered as extracellular can retain within the host cell by adapting intracellular bacterial lifestyle that includes the bacterial communities having biofilm-like properties. First, a murine model of infection was used to assess the bacterial communities for UPEC [85]. Type 1 pili in uropathogenic
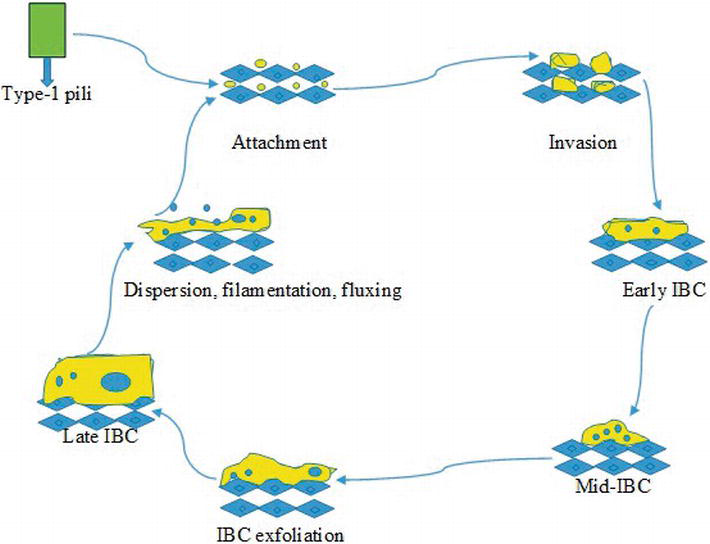
Figure 1.
Schematic diagram of the development of IBC cascade in uropathogenic
The amount of IBCs is found between 3 and 700 in an infected patient’s bladder—IBCs are composed of 104–105 bacterial cells [88]. There are numerous fibers surrounded on IBC bacteria that originate from the surface of pathogen and enclose pathogens in individualized sections. One of the main components present on the surface of IBCs called polysaccharide (sialic acid) that provides protection from the attack of immune system and environmental stress. The heterogeneous nature of IBCs, such as extracellular bacterial biofilm, composed of different subpopulation having distinct gene expression systems [89]. As IBCs expand, they induce the bacterial biofilm to cause interruption against cell membrane of host, producing a pod-like structure on the infected cell surface. Ultimately, UPEC detaches as filament or single rod at the IBC boundary and the infected cells are flux out into the lumen of bladder where can invade epithelial cells and restart the process through binding [85]. The inhibitor (SuIA) of cell division has been observed to be crucial for dispersal and filamentation of UPEC from the bacterial biofilm. The patients suffered from urinary tract infections (UTIs) are more likely observed with the UPEC filaments in their urine, but not in comparison with healthy controls [90].
The formation of IBC is prevented by intense molecular blockages and during acute infection—development of chronic cystitis—the IBC numbers are higher, representing the significance of intracellular pathways in the pathogenesis of UTIs [88]. The cycle of IBC is dependent on FimH, causing interruption in the expression of type-1 pili after invasion to host cell, and disrupts normal development of IBC due to attenuation of UPEC [54]. The two-component system (QseBC) is a key factor influencing curli expression, formation of IBC and type-1 pili. Some studies indicated that the intracellular pathway of UPEC is necessary for the TCA cycle completion [47]. The techniques such as qPCR and DNA microarray analyses interpreting the UPEC expression patterns within IBC pathogen exposed that acquisition of iron in bacteria is upregulated, representing the significance of system biomass formation [91]. While in clinical isolates of UPEC, the iron acquisition patterns are prevalent [92]. Moreover, the pathogen
4. Postantibiotic period: treatment strategy for biofilm
Broad-spectrum antibiotics are the drug of choice for the treatment of bacterial infections. Conventional antibiotics act as either killing the bacterial cell (bactericidal) or inhibiting the cell division (bacteriostatic). Numerous evidence shows that the use of antibiotics extensively causes damage to the host microbiota, producing a condition where invading bacteria can prevail and enhance the selective pressure against drug resistance [95]. Furthermore, surgery proceeded by administering antibiotics is highly successful in order to minimize the infection prophylactically. In certain cases, the perfect treatment of choice for foreign material associated with biofilm infections is the removal of infectious device. In some cases like pacemakers, cardiac implants and implantable prostheses, device removal is difficult [37]. Biofilm formation nature of bacteria that make them recalcitrant against different antimicrobial drugs is a result of prolonged treatment. There is a need for the irradiation or complete removal of these kinds of pathogens. Antibiotic resistance is not only due to increased resistance markers transmitted within the bacterial biofilm community, but also due to high metal ion concentration, low pH, and the presence of persistent cells that are metabolically inactive and inactivate the antibiotics [31]. All these characteristics make bacterial biofilm more tolerant/resistant to antimicrobial drugs up to 1000-fold more when compared to planktonic bacterial cells [96]. Therefore, an alternative strategy must be investigated to combat the antibiotic resistant strains and make them vulnerable to antimicrobial drugs. Here below, we have mentioned some of the recent developments in strategies that are considered to prevent formation of biofilm by bactericidal mechanism or targeting distinct developmental stages of biofilm (Figure 2).
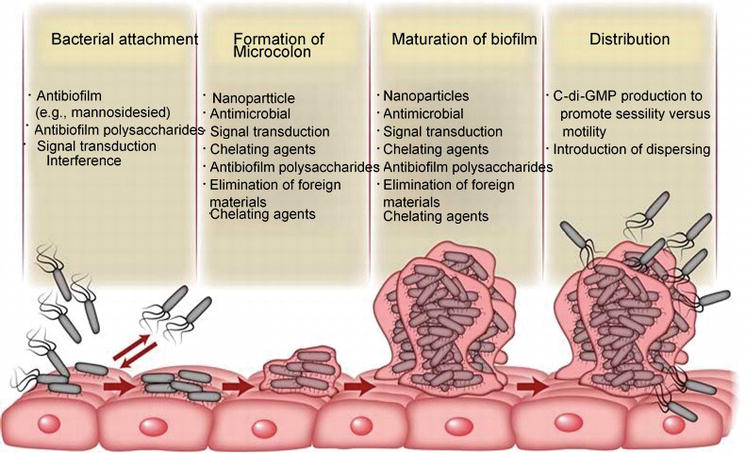
Figure 2.
Schematic diagram about the different stages in the development of biofilm and indicating the strategies to preventing and damaging the bacterial biofilm production at particular stages.
5. Bacterial killing strategies
5.1 Elimination of foreign material (indwelling devices) and abscess
There are studies that have reported that the presence of any foreign body (indwelling medical devices such as implants or prostheses or catheters) in low inoculums of
5.2 Phage therapy
An alternative approach to antibiotic treatment is phage therapy [98]. Phages are present in a wide range in the environment. It can be isolated easily and ubiquitous in nature. Their host ranges from specific to narrow, they are able to self-replicate, and therefore, a small dosage may be sufficient to disturb the host microorganisms. Furthermore, high mutation rate of phage facilitates adaptation as conforming bacterial host aggregate mutations to fix in a specific environment. Phage therapy has various advantages during lytic cycle phage that does not enter prophage cycle and rarely transfers or contains a virulence gene, thus causing destruction of bacterial cell rapidly. Many phages are associated with EPS degrading protein [99] or spread during stationary growth phase; these features allow to persist inside the bacterial biofilm [100].
5.3 Antimicrobial peptides
This is another alternative approach used for the improvement of new type of antimicrobial drug, usually produced by innate immune response mechanism [101]. Contrary to that, their mechanism of action and antimicrobial spectrum activity must be defined more accurately before applying as a therapeutic strategy. Cathelicidin, for instance, possesses most essential type of antibacterial peptides. The biofilm formation of multidrug-resistant
5.4 Silver nanoparticles
Many researchers have done research on the antimicrobial property of silver nanoparticles. Fey [37] found that the silver nanoparticles are the best alternative strategy to combat the bacterial biofilms. For example, antimicrobial agents (silver nanoparticles) have been incorporated with medical devices and have showed to inhibit the device-associated bacterial biofilms. Silver was frequently used as an antimicrobial agent for different pathogens over a 100 years; for instance, during World War 1, it was extensively used to sterilize the wound infections [104]. The antimicrobial activity of silver nanoparticles depends on the positively charged ions of metal and electrostatic interactions between negatively charged cell membrane of bacteria [105]. The thiol group in silver is the main cause of death in bacteria that play an important role in the inactivation of enzyme [106]. This is the reason why silver nanoparticles are increasingly used in response to various bacterial infections. The antimicrobial agents contain different properties such as high aspect ratios, nonimmunogenic, biocompatible, nonbiodegradable, ultralight weight, and easy cell membrane penetration. Due to such remarkable properties, we can apply silver nanoparticles in various applications such as infection therapy, gene therapy, and as antioxidants. The size of silver nanoparticles is typically smaller than 100 nm. The mechanism of action of silver nanoparticle is to interrupt the cell membrane of bacteria, generate the reactive oxygen species (ROS), interrupt the metabolic pathway, prevent the replication of DNA, disrupt the bacterial electron transport chain (ETC) [106], and release the toxic ions outside the bacterial cells that lead to the death of bacteria. There are large numbers of studies conducted regarding toxicity mechanism of silver nanoparticles in rabbits. There is a study that showed that silver nanoparticles inhibited bacterial biofilm formation against
5.5 Polysaccharides
Bacterial cell-to-cell interaction mediated by the exopolysaccharides is a serious threat to the formation of biofilm and stabilization. Mutants incapable to export or synthesize such exopolysaccharides are usually deficient in the formation of biofilm and adherence and hence are extremely sensitive to killing through host immune defenses and antimicrobial drugs [108]. Recent studies showed that certain bacterial exopolysaccharides destabilize or prevent biofilm formation by some pathogenic species. For instance, the existence of
5.6 Interference with signal transference
Many studies have been carried out on biofilm inhibition caused by interruption of the pathogen signaling cascades. This is possible provided that the two-component systems in bacteria establish a dominant means of translating and intercepting the environmental changes. Signal transduction inhibition system plays a critical role in response to antimicrobial therapy because of this type of signaling cascade interruption. Not only does it kill the pathogen, but it also interferes with the gene expression. Two-component system (QseBC) is the best alternative candidate for targeting the drugs, particularly in Gram-negative biofilm-forming pathogens [112]. QseC/QseB establishes a significant association between the bacterial environmental signaling and the host stress response. The pathogen (
5.7 Antimatrix agents
Apart from that, extracellular matrix with disrupting components is also very important to target the bacterial aggregates. Various observations exploited the inhibiting enzymes potentially involved in the modification or synthesis of cell wall-secreted or associated with EPS components. In these studies, use of engineered or naturally occurring enzyme and use of phage therapy as an enzyme delivery vehicle or to interrupt with matrix integrity by taking benefits from metal chelators have been recommended.
5.8 Chelating agents
Metal cations such as iron, magnesium, and calcium have been associated with stabilizing the matrix integrity [114]. Chelating agents indicated to cause interruption in the bacterial cell membrane stability besides disrupting the bacterial biomass structure [39].
5.9 Enzyme
The main mechanism of active dispersal of bacterial biofilm is through the formation of extracellular enzymes (proteins) that act on several structural components (such as exopolysaccharides, surface proteins, and extracellular DNA) of the extracellular polymeric substances. These enzymes play an important role in the cell separation from the bacterial biofilm colonies and facilitate their planktonic discharge into the environment [119]. Through purifying and isolating these enzymes, therapist can apparently add them to preformed bacterial biofilms exogenously at raised concentrations, in order to make biofilm-associated bacteria more susceptible to antimicrobials/antibiotics and to achieve interventional dispersal of biofilms. For this purpose, several classes of enzymes (specifically proteases, glycoside hydrolases, and deoxyribonucleases) have been explored for the eradication of bacterial biofilms [119]. The enzymes dispersin-B and DNase-I have gained greater attention as possible antibiofilm agents, especially in response to Gram-positive bacteria. The DNase effect depends on its capability to interrupt the eDNA that is established within the bacterial biomass structure [73]. The treatment of DNase prevents biofilm formation in
6. Conclusion
Currently, the removal of bacterial biofilm is the most challenging task for the clinicians and microbiologists. Antibiotics are not the best choice for the treatment of infections caused by bacteria forming biofilm. Biofilm formation allows the pathogen to adhere to the host surface under extreme condition and is resistant against a wide range of antibiotics. The choice of drug depends on the characteristics of the biofilm such as composition, age, solidity, and type of pathogens. These are the major components influencing the microbial susceptibility. As the bacterial biofilm matures, it enhances the accumulation of exopolymeric substance (EPS), attaches with the oxygen and nutrient gradients that effect bacterial growth rates and metabolism of cells, becomes impermeable, and reduces the activity of antimicrobial agents. This leads to resistance to most antibiotic regime. Therefore, novel potential therapeutic strategies should be considered to curb bacterial biofilm formation at specific stage without harming the pathogen. Antiadhesion and antimatrix agents are exciting strategies that may be used pending further investigation. extracellular polymeric substance deoxyribonuclease carbohydrate-binding protein deoxyribonucleic acid chaperone-usher pathway uropathogenic polyglucosamine small colony variants environmental deoxyribonucleic acid cyclic di-GMP alginate lyase toll-like receptor-4 intracellular bacterial communities urinary tract infections scanning electron microscope tricarboxylic acid quantitative polymerase chain reaction hospital-acquired infections colony forming unit multidrug resistant lipopolysaccharides electron transport chain reactive oxygen species enterohemorrhagic ethylene-diamine-tetra-acetic acid cystic fibrosisList of abbreviations
References
- 1.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews. Microbiology. 2004; 2 :95-108 - 2.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999; 284 :1318-1322 - 3.
O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annual Review of Microbiology. 2000; 54 :49-79 - 4.
Kolter R. Biofilms in lab and nature: A molecular geneticist’s voyage to microbial ecology. International Microbiology. 2010; 13 :1-7 - 5.
Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Molecular Microbiology. 1998;30 :285-293 - 6.
Prigent-Combaret C, Brombacher E, Vidal O, Ambert A, Lejeune P, Landini P, et al. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. Journal of Bacteriology. 2001;183 :7213-7223 - 7.
Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annual Review of Microbiology. 2003; 57 :677-701 - 8.
Lenz AP, Williamson KS, Pitts B, Stewart PS, Franklin MJ. Localized gene expression in Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 2008;74 :4463-4471 - 9.
Monds RD, O’Toole GA. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends in Microbiology. 2009; 17 :73-87 - 10.
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413 :860-864 - 11.
Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. Journal of Bacteriology. 2003;185 :1951-1957 - 12.
Schembri MA, Kjaergaard K, Klemm P. Global gene expression in Escherichia coli biofilms. Molecular Microbiology. 2003;48 :253-267 - 13.
Bagge N, Hentzer M, Andersen JB, Ciofu O, Givskov M, Hoiby N. Dynamics and spatial distribution of beta lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrobial Agents and Chemotherapy. 2004;48 :1168-1174 - 14.
Beloin C, Valle J, Latour-Lambert P, Faure P, Kzreminski M, Balestrino D, et al. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Molecular Microbiology. 2004;51 :659-674 - 15.
Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cellular Microbiology. 2004;6 :269-275 - 16.
Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. Journal of Bacteriology. 2008; 190 :4447-4452 - 17.
Klebensberger J, Birkenmaier A, Geffers R, Kjelleberg S, Philipp B. SiaA and SiaD are essential for inducing auto aggregation as a specific response to detergent stress in Pseudomonas aeruginosa . Environmental Microbiology. 2009;11 :3073-3086 - 18.
Flemming HC, Wingender J. The biofilm matrix. Nature Reviews. Microbiology. 2010; 8 :623-633 - 19.
Tielker D, Hacker S, Loris R, Strathmann M, Wingender J, Wilhelm S, et al. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151 :1313-1323 - 20.
Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Molecular Microbiology. 2006;59 :1229-1238 - 21.
Diggle SP, Stacey RE, Dodd C, Camara M, Williams P, Winzer K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa . Environmental Microbiology. 2006;8 :1095-1104 - 22.
Conrad A, Suutari MK, Keinanen MM, Cadoret A, Faure P, Mansuy-Huault L, et al. Fatty acids of lipid fractions in extracellular polymeric substances of activated sludge flocs. Lipids. 2003; 38 :1093-1105 - 23.
Sauer K, Cullen MC, Rickard AH, Zeef LA, Davies DG, Gilbert P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. Journal of Bacteriology. 2004;186 :7312-7326 - 24.
Gjermansen M, Ragas P, Sternberg C, Molin S, Tolker Nielsen T. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environmental Microbiology. 2005;7 :894-906 - 25.
Ma L, Conover M, Lu H, Parsek MR, Bayles K, Wozniak DJ. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathogens. 2009;5 :e1000354 - 26.
Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrobial Agents and Chemotherapy. 2003;47 :317-323 - 27.
Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrobial Agents and Chemotherapy. 2005;49 :2467-2473 - 28.
Mai-Prochnow A, Lucas-Elio P, Egan S, Thomas T, Webb JS, Sanchez-Amat A, et al. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteria. Journal of Bacteriology. 2008; 190 :5493-5501 - 29.
Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc). 2005; 70 :267-274 - 30.
Domka J, Lee J, Bansal T, Wood TK. Temporal gene expression in Escherichia coli K-12 biofilms. Environmental Microbiology. 2007;9 :332-346 - 31.
Lewis K. Multidrug tolerance of biofilms and persister cells. Current Topics in Microbiology and Immunology. 2008; 322 :107-131 - 32.
Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infection and Immunity. 2006;74 :4849-4855 - 33.
Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993; 341 :1065-1069 - 34.
Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa andBurkholderia cepacia . Microbiological Reviews. 1996;60 :539-574 - 35.
Kuramitsu HK, Wang BY. The whole is greater than the sum of its parts: Dental plaque bacterial interactions can affect the virulence properties of cariogenic Streptococcus mutans . American Journal of Dentistry. 2011;24 :153-154 - 36.
Venditti M, Biavasco F, Varaldo PE, Macchiarelli A, De Biase L, Marino B, et al. Catheter-related endocarditis due to glycopeptide-resistant Enterococcus faecalis in a transplanted heart. Clinical Infectious Diseases. 1993;17 :524-525 - 37.
Fey PD. Modality of bacterial growth presents unique targets: How do we treat biofilm-mediated infections? Current Opinion in Microbiology. 2010; 13 :610-615 - 38.
Foxman B. The epidemiology of urinary tract infection. Nature Reviews. Urology. 2010; 7 :653-660 - 39.
Donlan RM. Biofilms: Microbial life on surfaces. Emerging Infectious Diseases. 2002; 8 :881-890 - 40.
Beloin C, Roux A, Ghigo JM. Escherichia coli biofilms. Current Topics in Microbiology and Immunology. 2008;322 :249-289 - 41.
Toutain CM, Caizza NC, Zegans ME, O’Toole GA. Roles for flagellar stators in Biofilm formation by Pseudomonas aeruginosa . Research in Microbiology. 2007;158 :471-477 - 42.
Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. Journal of Bacteriology. 2007;189 :4418-4424 - 43.
Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Molecular Microbiology. 2003a;50 :61-68 - 44.
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Molecular Microbiology. 2003b;48 :1511-1524 - 45.
Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Molecular Microbiology. 1999;34 :586-595 - 46.
Schmidt J, Musken M, Becker T, Magnowska Z, Bertinetti D, Moller S, et al. The Pseudomonas aeruginosa chemotaxis methyltransferase CheR1 impacts on bacterial surface sampling. PLoS One. 2011;6 :e18184 - 47.
Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli . Molecular Microbiology. 2011;80 :1516-1529 - 48.
Dunne WM Jr. Bacterial adhesion: Seen any good biofilms lately? Clinical Microbiology Reviews. 2002; 15 :155-166 - 49.
Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, et al. Induction and evasion of host defenses by type-1-piliated uropathogenic Escherichia coli . Science. 1998;282 :1494-1497 - 50.
Hung CS, Bouckaert J, Hung D, Pinkner J, Widberg C, DeFusco A, et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Molecular Microbiology. 2002;44 :903-915 - 51.
Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-Mediated bacterial invasion of bladder epithelial cells. The EMBO Journal. 2000; 19 :2803-2812 - 52.
Nilsson LM, Yakovenko O, Tchesnokova V, Thomas WE, Schembri MA, Vogel V, et al. The cysteine bond in the Escherichia coli FimH adhesin is critical for adhesion under flow conditions. Molecular Microbiology. 2007;65 :1158-1169 - 53.
Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infection and Immunity. 2007;75 :52-60 - 54.
Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cellular Microbiology. 2007;9 :2230-2241 - 55.
Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, et al. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: A comparative genomics approach. Proceedings of the National Academy of Sciences. 2006;103 :5977-5982 - 56.
Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proceedings of the National Academy of Sciences. 2009; 106 :22439-22444 - 57.
Hasman H, Chakraborty T, Klemm P. Antigen-43 mediated auto aggregation of Escherichia coli is blocked by fimbriation. Journal of Bacteriology. 1999;181 :4834-4841 - 58.
Uhlich GA, Cooke PH, Solomon EB. Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Applied and Environmental Microbiology. 2006;72 :2564-2572 - 59.
Mohamed JA, Teng F, Nallapareddy SR, Murray BE. Pleiotrophic effects of 2 Enterococcus faecalis sagA-like genes, salA and salB, which encode proteins that are antigenic during human infection, on biofilm formation and binding to collagen type I and fibronectin. The Journal of Infectious Diseases. 2006;193 :231-240 - 60.
Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, et al. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Applied and Environmental Microbiology. 2001;67 :4538-4545 - 61.
Kemp KD, Singh KV, Nallapareddy SR, Murray BE. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infection and Immunity. 2007;75 :5399-5404 - 62.
Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang X, Holm A, et al. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathogens. 2010;6 :e1001010 - 63.
Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nature Chemical Biology. 2009;5 :913-919 - 64.
Wang X, Preston JF 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. Journal of Bacteriology. 2004;186 :2724-2734 - 65.
Harmsen M, Yang L, Pamp SJ, Tolker-Nielsen T. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunology and Medical Microbiology. 2010;59 :253-268 - 66.
Wittschier N, Lengsfeld C, Vorthems S, Stratmann U, Ernst JF, Verspohl EJ, et al. Large molecules as anti-adhesive compounds against pathogens. The Journal of Pharmacy and Pharmacology. 2007; 59 :777-786. [PubMed] - 67.
Starkey M, Hickman JH, Ma L, Zhang N, DeLong S, Hinz A, et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. Journal of Bacteriology. 2009;191 :3492-3503 - 68.
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa . Microbiology. 2007;153 :1318-1328 - 69.
Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Molecular Microbiology. 2006;59 :1114-1128 - 70.
Barken KB, Pamp SJ, Yang L, Gjermansen M, Bertrand JJ, Klausen M, et al. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environmental Microbiology. 2008;10 :2331-2343 - 71.
Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. Journal of Bacteriology. 2008;190 :5690-5698 - 72.
Thomas VC, Hiromasa Y, Harms N, Thurlow L, Tomich J, Hancock LE. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis . Molecular Microbiology. 2009;72 :1022-1036 - 73.
Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, et al. Contribution of autolysin and Sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infection and Immunity. 2009;77 :3626-3638 - 74.
Hong SH, Lee J, Wood TK. Engineering global regulator Hha of Escherichia coli to control biofilm dispersal. Microbial Biotechnology. 2010;3 :717-728 - 75.
Rowe MC, Withers HL, Swift S. Uropathogenic Escherichia coli forms biofilm aggregates under iron restriction that disperse upon the supply of iron. FEMS Microbiology Letters. 2010;307 :102-109 - 76.
Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiology and Molecular Biology Reviews. 2009; 73 :310-347 - 77.
Kaplan JB. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. Journal of Dental Research. 2010; 89 :205-218 - 78.
Wood TK, Hong SH, Ma Q. Engineering biofilm formation and dispersal. Trends in Biotechnology. 2010; 29 :87-94 - 79.
Boyd A, Chakrabarty AM. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa . Applied and Environmental Microbiology. 1994;60 :2355-2359 - 80.
Wang X, Dubey AK, Suzuki K, Baker CS, Babitzke P, Romeo T. CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli . Molecular Microbiology. 2005;56 :1648-1663 - 81.
Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, et al. Cell death in Pseudomonas aeruginosa biofilm development. Journal of Bacteriology. 2003;185 :4585-4592 - 82.
Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, et al. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009; 325 :1552-1555 - 83.
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010; 328 :627-629 - 84.
Kolodkin-Gal I, Cao S, Chai L, Bottcher T, Kolter R, Clardy J, et al. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell. 2012; 149 :684-692 - 85.
Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proceedings of the National Academy of Sciences. 2004;101 :1333-1338 - 86.
Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli . PLoS Pathogens. 2007;3 :e100 - 87.
Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nature Medicine. 2007;13 :625-630 - 88.
Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infection and Immunity. 2011;79 :4250-4259 - 89.
Anderson GG, Martin SM, Hultgren SJ. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes and Infection. 2004; 6 :1094-1101 - 90.
Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Medicine. 2007; 4 :e329 - 91.
Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. The Journal of Biological Chemistry. 2007;282 :21259-21267 - 92.
Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli . PLoS Pathogens. 2009;5 :e1000305 - 93.
Hansen DS, Gottschau A, Kolmos HJ. Epidemiology of Klebsiella bacteremia: A case control study usingEscherichia coli bacteremia as control. The Journal of Hospital Infection. 1998;38 :119-132 - 94.
Garcia-Medina R, Dunne WM, Singh PK, Brody SL. Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infection and Immunity. 2005;73 :8298-8305 - 95.
Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of Clinical Investigation. 2010;120 :4332-4341 - 96.
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents. 2010; 35 :322-332 - 97.
Zimmerli W, Lew PD, Waldvogel FA. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. The Journal of Clinical Investigation. 1984; 73 (4):1191-1200 - 98.
Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends in Microbiology. 2009; 17 :66-72 - 99.
Sutherland IW, Hughes KA, Skillman LC, Tait K. The interaction of phage and biofilms. FEMS Microbiology Letters. 2004; 232 :1-6 - 100.
Burrowes B, Harper DR, Anderson J, McConville M, Enright MC. Bacteriophage therapy: Potential uses in the control of antibiotic-resistant pathogens. Expert Review of Anti-Infective Therapy. 2011; 9 :775-785 - 101.
Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: More than just microbicidal. Trends in Immunology. 2002; 23 :291-296 - 102.
Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, et al. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides. 2011; 32 :1807-1814 - 103.
Kharidia R, Liang JF. The activity of a small lytic peptide PTP-7 on Staphylococcus aureus biofilms. Journal of Microbiology. 2011; 49 :663-668 - 104.
Chen X, Schluesener HJ. Nanosilver: A nanoproduct in medical application. Toxicology Letters. 2008; 176 :1-12 - 105.
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007; 3 :95-101 - 106.
Feng QL, Wu J, Chen GQ , Cui FZ, Kim TN, Kim JO. A mechanistic study of the anti-bacterial effect of silver ions on Escherichia coli andStaphylococcus aureus . Journal of Biomedical Materials Research. 2000;52 :662-668 - 107.
Secinti KD, Ozalp H, Attar A, Sargon MF. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. Journal of Clinical Neuroscience. 2011; 18 :391-395 - 108.
Rendueles O, Kaplan JB, Ghigo JM. Antibiofilm polysaccharides. Environmental Microbiology. 2012; 15 (2):334-346. DOI: 10.1111/j.14622920.2012.02810.x - 109.
Pihl M, Davies JR, Chavez de Paz LE, Svensater G. Differential effects of Pseudomonas aeruginosa on biofilm formation by different strains ofStaphylococcus epidermidis . FEMS Immunology and Medical Microbiology. 2010;59 :439-446 - 110.
Kim Y, Oh S, Kim SH. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochemical and Biophysical Research Communications. 2009;379 :324-329. [PubMed] - 111.
Zinger-Yosovich KD, Gilboa-Garber N. Blocking of Pseudomonas aeruginosa andRalstonia solanacearum lectins by plant and microbial branched polysaccharides used as food additives. Journal of Agricultural and Food Chemistry. 2009;57 :6908-6913. [PubMed] - 112.
Wang X, Wang Q , Yang M, Xiao J, Liu Q , Wu H, et al. QseBC controls flagellar motility, fimbrial hemagglutination and intracellular virulence in fish pathogen Edwardsiella tarda . Fish & Shellfish Immunology. 2011;30 :944-953 - 113.
Prüß BM. Involvement of Two-Component Signaling on Bacterial Motility and Biofilm Development. Journal of Bacteriology. 2017; 199 (18):e00259-e00217 - 114.
Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Current Opinion in Infectious Diseases. 2008; 21 :385-392 - 115.
Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrology, Dialysis, Transplantation. 2006; 21 :2247-2255 - 116.
Bookstaver PB, Williamson JC, Tucker BK, Raad II, Sherertz RJ. Activity of novel antibiotic lock solutions in a model against isolates of catheter-related bloodstream infections. The Annals of Pharmacotherapy. 2009; 43 :210-219 - 117.
Chatzinikolaou I, Zipf TF, Hanna H, Umphrey J, Roberts WM, Sherertz R, et al. Minocycline-ethylene-diaminetetraacetate lock solution for the prevention of implantable port infections in children with cancer. Clinical Infectious Diseases. 2003; 36 :116-119 - 118.
Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ. A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infection Control and Hospital Epidemiology. 2005; 26 :520-524 - 119.
Fleming D, Rumbaugh KP. Approaches to dispersing medical biofilm. Microorganisms. 2017; 5 :15. DOI: 10.3390/microorganisms5020015 - 120.
Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proceedings of the National Academy of Sciences. 2007; 104 :11197-11202