Regulatory limit of selected heavy metals.
Abstract
Several heavy metals are found naturally in the earth crust and are exploited for various industrial and economic purposes. Among these heavy metals, a few have direct or indirect impact on the human body. Some of these heavy metals such as copper, cobalt, iron, nickel, magnesium, molybdenum, chromium, selenium, manganese and zinc have functional roles which are essential for various diverse physiological and biochemical activities in the body. However, some of these heavy metals in high doses can be harmful to the body while others such as cadmium, mercury, lead, chromium, silver, and arsenic in minute quantities have delirious effects in the body causing acute and chronic toxicities in humans. The focus of this chapter is to describe the various mechanism of intoxication of some selected heavy metals in humans along with their health effects. Therefore it aims to highlight on biochemical mechanisms of heavy metal intoxication which involves binding to proteins and enzymes, altering their activity and causing damage. More so, the mechanism by which heavy metals cause neurotoxicity, generate free radical which promotes oxidative stress damaging lipids, proteins and DNA molecules and how these free radicals propagate carcinogenesis are discussed. Alongside these mechanisms, the noxious health effects of these heavy metals are discussed.
Keywords
- heavy metals
- toxicity
- neurotoxicity
- carcinogenesis
- free radicals
- health effects
1. Introduction
Metals are natural constituents that exist in the ecosystem. They are substances with high electrical conductivity which voluntarily lose their electrons to form cations. Metals are found all over the earth including the atmosphere, earth crust, water bodies, and can also accumulate in biological organisms including plants and animals. Among the 35 natural existing metals, 23 possess high specific density above 5 g/cm3 with atomic weight greater than 40.04 and are generally termed heavy metals [1, 2]. Theses metals generally termed heavy metals include: antimony, tellurium, bismuth, tin, thallium, gold, arsenic, cerium, gallium, cadmium, chromium, cobalt, copper, iron, lead, mercury, manganese, nickel, platinum, silver, uranium, vanadium, and zinc [1, 2]. This category of metals termed heavy metals have not only been known for their high density but most importantly for their adverse effects to the ecosystem and living organisms [3]. Some of these heavy metals such as cobalt, chromium, copper, magnesium, iron, molybdenum, manganese, selenium, nickel and zinc are essential nutrients that are required for various physiological and biochemical functions in the body and may result to deficiency diseases or syndromes if not in adequate amounts [4] but in large doses they may cause acute or chronic toxicities.
These heavy metals are distributed in the environment through several natural processes such as volcanic eruptions, spring waters, erosion, and bacterial activity, and through anthropogenic activities which include fossil fuel combustion, industrial processes, agricultural activities as well as feeding [5]. These heavy metals do bioaccumulate in living organisms and the human body through various processes causing adverse effects. In the human body, these heavy metals are transported and compartmentalized into body cells and tissues binding to proteins, nucleic acids destroying these macromolecules and disrupting their cellular functions. As such, heavy metal toxicity can have several consequences in the human body. It can affect the central nervous function leading to mental disorder, damage the blood constituents and may damage the lungs, liver, kidneys and other vital organs promoting several disease conditions [6]. Also, long term accumulation of heavy metals in the body may result in slowing the progression of physical, muscular and neurological degenerative processes that mimic certain diseases such as Parkinson’s disease and Alzheimer’s disease [6]. More so, repeated long-term contact with some heavy metals or their compounds may even damage nucleic acids, cause mutation, mimic hormones thereby disrupting the endocrine and reproductive system and eventually lead to cancer [7].
This chapter will highlight on the various sources of heavy metals and the processes that promote their exposure and bioaccumulation in the human body. More focus will be laid on the various mechanisms that lead to heavy metal toxicity with emphasis on macromolecule and cellular damages, carcinogenesis, neurotoxicity and the molecular basis for their noxious effects. The various toxic effects along with the signs and symptoms of some heavy metals in the human body will be discussed.
2. Sources of heavy metal exposure to humans
Heavy metals are naturally present in our environment. They are present in the atmosphere, lithosphere, hydrosphere and biosphere [8]. Although these heavy metals are present in the ecosystem, their exposure to humans is through various anthropogenic activities of man. In the earth crust, these heavy metals are present in ores which are recovered during mining activities as minerals. In most ores heavy metals such as arsenic, iron, lead, zinc, gold, nickel, silver and cobalt exist as sulfides while others such as manganese, aluminum, selenium gold, and antimony exist as oxides. Certain heavy metals such as copper, iron and cobalt can exist both as sulfide and oxide ores. Some sulfides may contain two or more heavy metals together such as chalcopyrite, (CuFeS2) which contains both copper and iron. During these mining activities, heavy metals are released from the ore and scattered in open in the environment; left in the soil, transported by air and water to other areas. Furthermore, when these heavy metals are used in the industries for various industrial purposes, some of these elements are released into the air during combustion or into the soil or water bodies as effluents. More so, the industrial products such as paints, cosmetics, pesticides, and herbicides also serve as sources of heavy metals. Heavy metals may be transported through erosion, run-off or acid rain to different locations on soils and water bodies. As reviewed from [9], the sources of specific heavy metals are described below.
2.1 Arsenic
Arsenic is the 20th most abundant element on earth and the 33rd on the periodic table. The inorganic forms such as arsenite and arsenate compounds are lethal to humans and other organisms in the environment. Humans get in contact with arsenic through several means which include industrial sources such as smelting and microelectronic industries. Drinking water may be contaminated with arsenic which is present in wood preservatives, herbicides, pesticides, fungicides and paints [10].
2.2 Lead
Lead is a slightly bluish, bright silvery metal in a dry atmosphere. The main sources of lead exposure include drinking water, food, cigarette, industrial processes and domestic sources. The industrial sources of lead include gasoline, house paint, plumbing pipes, lead bullets, storage batteries, pewter pitchers, toys and faucets [11]. Lead is released into the atmosphere from industrial processes as well as from vehicle exhausts. Therefore, it may get into the soil and flow into water bodies which can be taken up by plants and hence human exposure of lead may also be through food or drinking water [12].
2.3 Mercury
The metallic mercury is a shiny silver-white, odorless liquid metal which becomes colorless and odorless gas upon heating. Mercury is used in producing dental amalgams, thermometers and some batteries. Also, it can be found in some chemical, electrical-equipment, automotive, metal-processing, and building industries. Mercury can exist in a gaseous form thus it can be inhaled. Other forms of mercury contamination in humans may be through anthropogenic activities such as municipal wastewater discharges, agriculture, incineration, mining, and discharges of industrial wastewater [13].
2.4 Cadmium
This metal is mostly used in industries for the production of paints, pigments alloys, coatings, batteries as well as plastics. Majority of cadmium, about three-fourths is used as electrode component in producing alkaline batteries. Cadmium is emitted through industrial processes and from cadmium smelters into sewage sludge, fertilizers, and groundwater which can remain in soils and sediments for several decades and taken up by plants. Therefore, significant human exposure to cadmium can be by the ingestion of contaminated foodstuffs especially cereals, grains, fruits and leafy vegetables as well as contaminated beverages [14, 15]. Also, humans may get exposed to cadmium by inhalation through incineration of municipal waste.
2.5 Chromium
Chromium is a metal that is present in petroleum and coal, chromium steel, pigment oxidants, fertilizers, catalyst, oil well drilling and metal plating tanneries. Chromium is extensively used in industries such as wood preservation, electroplating, metallurgy, production of paints and pigments, chemical production, tanning, and pulp and paper production. These industries play a major role in chromium pollution with an adverse effect on biological and ecological species [16]. Following the anthropogenic activities by humans, disposal of sewage and use of fertilizers may lead to the release of chromium into the environment [16]. Therefore, these industrial and agricultural practices increase the environmental contamination of chromium. Environmental pollution by chromium has been mostly by the hexavalent chromium in recent years [17].
2.6 Copper
This is a heavy metal which is used in industries to produce copper pipes, cables, wires, copper cookware, etc. It is also used to make copper intrauterine devices and birth control pills. Copper in the form of copper sulfate is added to drinking water and swimming pools [18]. Due to man’s anthropogenic and industrial activities, it can accumulate in the soil and up taken by plants. As such, copper is present in some nuts, avocado, wheat germ and bran etc.
2.7 Manganese
This metal is added to gasoline as methylcyclopentadienyl manganese tricarbonyl (MMT) and thus, gasoline fumes contain a very toxic form of manganese [19].
2.8 Nickel
It is used in the production of batteries, nickel-plated jewelry, machine parts, nickel plating on metallic objects, manufacture of steel, cigarette smoking, wire, electrical parts, etc. Also, it can be found in food stuff such as imitation whip cream, unrefined grains and cereals, commercial peanut butter, hydrogenated vegetable oils, as well as contaminated alcoholic beverages [19]. The various sources of heavy metals are summarized in Figure 1.
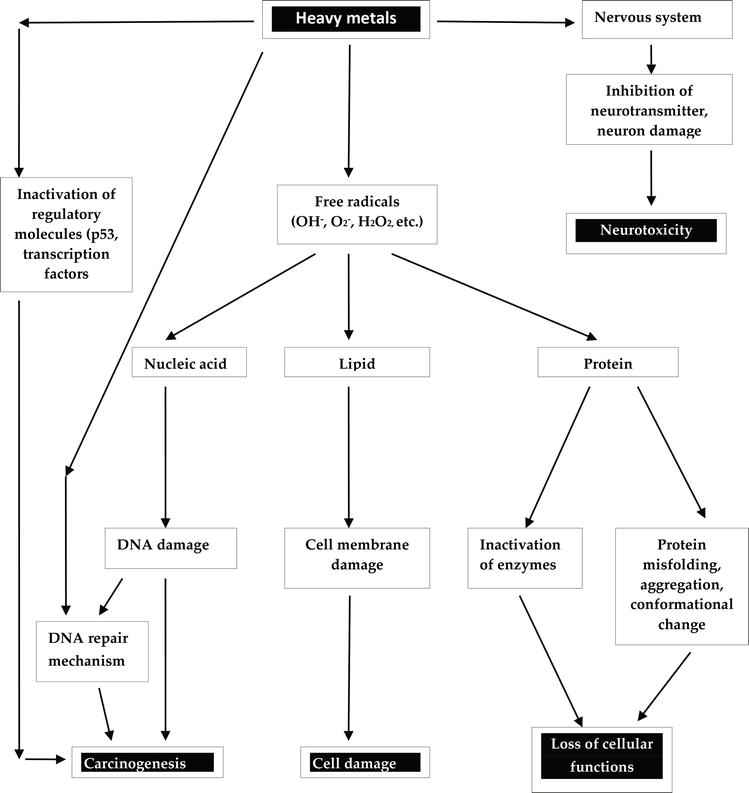
Figure 1.
Pathway of heavy metals sources and exposure to humans.
3. Route of exposure, bio-uptake and bioaccumulation of heavy metals in humans
Humans may directly get in contact with heavy metals by consuming contaminated food stuffs, sea animals, and drinking of water, through inhalation of polluted air as dust fumes, or through occupational exposure at workplace [20]. The contamination chain of heavy metals almost usually follows this cyclic order: from industry, to the atmosphere, soil, water and foods then human [8]. These heavy metals can be taken up through several routes. Some heavy metals such as lead, cadmium, manganese, arsenic can enter the body through the gastrointestinal route; that is, through the mouth when eating food, fruits, vegetables or drinking water or other beverages. Others can enter the body by inhalation while others such as lead can be absorbed through the skin.
Most heavy metals are distributed in the body through blood to tissues [21]. Lead is carried by red blood cells to the liver and kidney and subsequently redistributed to the teeth, bone and hair mostly as phosphate salt [20]. Cadmium initially binds to blood cells and albumin, and subsequently binds to metallothionein in kidney and liver tissue. Following its distribution from blood to the lungs, manganese vapor diffuses across the lung membrane to the Central nervous system (CNS). Organic salts of manganese which are lipid soluble are distributed in the intestine for fecal elimination while inorganic manganese salts which are water soluble are distributed in plasma and kidney for renal elimination. Arsenic is distributed in blood and accumulates in heart, lung, liver, kidney, muscle and neural tissues and also in the skin, nails and hair. The regulatory limit for some selected heavy metals is shown in Table 1.
| Heavy metals | EPA limits in drinking water (ppm) | OSHA limit in workplace air (mg) | FDA limit in bottled water/food (ppm) |
|---|---|---|---|
| Arsenic | 0.01 | 10 | – |
| Barium | 2.0 | 0.5 | – |
| Cadmium | 0.005 | 5 | 0.005 |
| Chromium | 0.1 | 1 | 1 |
| Lead | 0.015 | 0.15 | – |
| Mercury | 0.002 | 0.1 | 1 |
| Selenium | 0.05 | 0.2 | – |
| Silver | 0.0001 | 0.01 | – |
| Zinc | 5 | 5 | – |
Table 1.
ppm, parts per million; mg, milligram; EPA, Environmental Protection Agency; OSHA, Occupational Safety and Health Administration; FDA, Food and Drug Administration.
4. Mechanism of heavy metal toxicity
4.1 Heavy metal-induced oxidative stress and oxidation of biological molecules
Certain heavy metals are known to generate free radicals which may lead to oxidative stress and cause other cellular damages (see [22] for review). The mechanism of free radical generation is specific to the type of heavy metal.
4.1.1 Iron
Iron is a useful heavy metal in the human body as it is a constituent of certain biological molecules like the hemoglobin and involved in various physiological activities. However, in its free state, iron is one of the heavy metals generally known to generate hydroxyl radical (OH•) as shown below by the Fenton reaction.
Net reaction (Haber-Weiss reaction):
In addition to the above reactions, the following reactions below can also occur:
Hydroxyl radical (OH•) is the most common free radical generated by the oxidation of iron. OH• is capable of reacting with biological molecules such as proteins, lipids and DNA damaging them. When OH• reacts with guanine, a nitrogenous base of nucleic acids, it leads to the generation of 8-oxo-7,8-dihydro-20-deoxyguanosine (8-oxo-dG) and 2,6-diamino-5-formamido-4-hydroxypyrimidine (FAPy-G), in which the former is a good marker for oxidative damage [23].
It is well documented that metal-induced generation of oxygen reactive species can attack polyunsaturated fatty acid such as phospholipids. The first of such observation was first presented by Bucher et al. [24] who showed that iron-generated OH• can oxidize lipid membranes through a process known as lipid peroxidation. Following his experimental observations, he proposed the following mechanism:
Steps of lipid peroxidation:
At the initiation stage, the radical (R•)/OH• attacks the lipid membrane to form a radial lipid. This radical lipid further propagates the formation of peroxyl lipid radical by reacting with dioxygen molecule or with a lipid. This reaction further promotes damage of the lipid molecule. At the termination stage, two radical lipid molecules and/or with a peroxyl lipid radical reacts to form a stable lipid molecule. The major aldehyde product of lipid peroxidation is malondialdehyde and it serves as a marker for lipid peroxidation.
Generally, proteins are not easily damaged by H2O2 and other simple oxidants unless transition metals are present. Thus, protein damaged are usually metal-catalyzed and involves oxidative scission, bityrosine cross links, loss of histidine residues, the introduction of carbonyl groups, and the formation of protein-centered alkyl (R•), alkoxyl (RO•) and alkylperoxyl (ROO•) radicals [25].
4.1.2 Copper
Copper ions have been identified to participate in the formation of reactive oxygen species (ROS) as cupric (Cu2+) and cuprous (Cu1+) which can participate in oxidation and reduction reactions. The Cu2+ in the presence of biological reductants such as glutathione (GSH) or ascorbic acid can be reduced to Cu+ which is capable of catalyzing the decomposition of H2O2 to form OH•
The OH• radical formed is capable of reacting with several biomolecules. Experimental studies confirmed that copper is also capable of inducing DNA strand breaks and oxidation of bases
4.1.3 Chromium
Chromium (Cr), particularly Cr4+ has been shown in
4.1.4 Cobalt
Cobalt (Co), particularly Co2+ has been shown to generate superoxide (•O2−) from the decomposition of H2O2 [30].
4.1.5 Vanadium
Vanadium is a heavy metal that occurs in various oxidative states and has been shown to generate free radical. In the plasma, vanadium (V) is rapidly reduced to vanadium (IV) by NADPH and ascorbic acid antioxidants which bind to plasma proteins for transportation [31].
More so under physiological conditions at approximately pH of 7, V(IV) can generate OH• from the decomposition of H2O2 according to the Fenton reaction.
4.1.6 Arsenic
Arsenic has also been shown to generate free radicals such as superoxide (O2•–), singlet oxygen (1O2), nitric oxide (NO•), hydrogen peroxide (H2O2), the peroxyl radical (ROO•) [32], dimethylarsinic peroxyl radicals ((CH3)2AsOO•) and also the dimethylarsinic radical ((CH3)2As•) [33] in some studies though the mechanism for the generation of all these reactive species remains unclear.
4.2 Heavy metal-induced carcinogenesis
Some heavy metals are known to have carcinogenic effect. Several signaling proteins or cellular regulatory proteins that participate in apoptosis, cell cycle regulation, DNA repair, DNA methylation, cell growth and differentiation are targets of heavy metals [34]. Thus, heavy metals may induce carcinogenic effect by targeting a number of these proteins. More so, the carcinogenic effects of certain heavy metals have been related to the activation of redox-sensitive transcription factors such as AP-1, NF-κB and p53 through the recycling of electrons by antioxidant network. These transcription factors control the expression of protective genes that induce apoptosis, arrest the proliferation of damaged cells, repair damaged DNA and power the immune system [22]. Metal signalization of transcription factor AP-1 and NF-κB has been observed in the mitogen-activated protein (MAP) kinase pathways where the nuclear transcription factor NF-κB, is involved in controlling inflammatory responses while AP-1 is involved in cell growth and differentiation [22]. The p53 protein is an important protein in cell division as it guards a cell-cycle checkpoint and control cell division [35]. Inactivation of p53 allows uncontrolled cell division and thus p53 gene disruption has been associated with most human cancers. Also, AP-1 and NF-κB family of transcription factors are involved in both cell proliferation and apoptosis, and also regulate p53. Heavy metals generated free radicals inside the cell selectively activates these transcription factors and thus, may suggest that cell proliferation or cell death may be related to the exposure to carcinogenic metals. There exist various mechanisms of heavy metal-induced carcinogenesis.
4.2.1 Arsenic
Arsenic-induced carcinogenic mechanisms include epigenetic alterations, damage to the dynamic DNA maintenance system and generation of ROS [36, 37]. Alterations of histones, DNA methylation, and miRNA are the key epigenetic changes induced by arsenic which have shown to possess potentials to cause malignant growth [37]. In vitro studies have shown arsenic to alter the expression of p53 protein which also led to decreased expression of p21, one downstream target [38]. Arsenic compounds have been shown in an
4.2.2 Lead
The mechanism of lead-induced carcinogenic process is postulated to induce DNA damage, disrupt DNA repair system and cellular tumor regulatory genes through the generation of ROS [42]. Studies have supported with evidence that ROS generation by lead is key in altering chromosomal structure and sequence [42]. Lead can disrupt transcription processes by replacing zinc in certain regulatory proteins [42].
4.2.3 Mercury
Little is known on the potential of mercury to act as a mutagen or carcinogen. However, the proposed mechanism of mercury-induced cancer is through the generation of free radicals inducing oxidative stress thereby damaging biomolecules. Mercury has been shown to induce malignant growth through the generation of free radicals as well as disruption of DNA molecular structure, the repair and maintenance system [43].
4.2.4 Nickel
Nickel has an extensive range of carcinogenic mechanisms which include regulation of transcription factors, controlled expression of certain genes and generation of free radicals. Nickel has been shown to be implicated in regulating the expression of specific long non-coding RNAs, certain mRNAs and microRNAs. Nickel can promote methylation of promoter and induce the down regulation of maternally expressed gene 3 (MEG3) thereby upregulating hypoxia-inducible factor-1α, two proteins which are known to be implicated in carcinogenesis [44]. It has also been demonstrated that nickel can generate free radicals, which contributes to carcinogenic processes [45].
4.2.5 Cadmium
Cadmium has been implicated in promoting apoptosis, oxidative stress, DNA methylation and DNA damage.
4.2.6 Iron
The main cause of cancer due to iron intoxication is through the generation of free radicals. A school of thought produced a mechanism for iron-induced cancer whereby bile acids (deoxycholic acid), iron(II) complexes, vitamins K and oxygen interact to generate free radicals which induced oncogenic effect in the colon.
4.3 Heavy metal-induced neurotoxicity
Some heavy metals such as lead and manganese may affect the brain and cause neurological toxicity as reviewed from [46].
4.3.1 Lead
Lead toxicity is targeted towards the memory and learning processes of the brain and can be mediated through three processes. Lead can impair learning and memory in the brain by inhibiting the N-methyl-d-aspartate receptor (NMDAR) and can block neurotransmission by inhibit neurotransmitter release, block the neuronal voltage-gated calcium (Ca2+) channels (VGCCs) and reduce the expression of brain-derived neurotrophic factor (BDNF).
4.3.2 Inhibition of NMDAR
The NMDAR is known to enhance learning and memory mediated by the hippocampus [47] as this has been confirmed in animal studies in which animals exposed to lead during its developmental process exhibit similar learning deficits comparable to those with the absence or impaired NMDARs [48, 49]. In the hippocampus, NMDAR is a neural receptor which consists of two or more subunits; an obligatory NR1 subunit and one or more subunits from the NR2 particularly NR2A, NR2B and NR3 families. Lead has been shown to be a potent, non-competitive antagonist of the NMDAR [50, 51, 52, 53], preferentially with high affinity at a regulatory site on the NR2A subunit [54]. This has been further supported in electrophysiological studies in which recombinant receptors for the subunits have shown NR2A-NMDARs to be more potently inhibited by lead than NR2B-NMDARs [55]. More so, lead has been shown to decrease the content of NR2A in the hippocampus and also alter the expression of NR1 spliced variants [56, 57] suggesting lead exposure disrupts the normal ontogeny of NMDAR.
4.3.3 Reduction of neurotransmission
Lead can decrease neurotransmission as long term exposure of rats to low levels of lead has shown reduction in the release of Ca2+-dependent glutamate and γ-aminobutyric acid (GABA) in the hippocampus [58, 59]. This indicates dysfunction of presynaptic neuron signalization in the hippocampus as a result of lead exposure [60]. More so, lead exposure also impairs two postsynaptic currents; inhibitory post synaptic currents (IPSCs) and excitatory post synaptic currents (EPSCs) which are dependent on the release of presynaptic neurotransmitter such as glutamate and GABA. Thus, lead exposure leads to reductions in IPSCs and EPSCs indicating a deficit in glutamatergic and GABAergic neurotransmission systems. Also, lead has been shown to reduce the expression of key presynaptic proteins such as synaptobrevin (Syb) and synaptophysin (Syn) involved in vesicular neurotransmitter release [59, 60]. Lead can disrupt neurotransmission by inhibiting the neuronal voltage-gated calcium (Ca2+) channels (VGCCs) [61]. Thus, inhibition of presynaptic VGCCs may reduce the influx of Ca2+ which is required for fast release of vesicular neurotransmitter thus interfering with neurotransmission. It is now suggested that inhibition of either NMDARs or VGCCs by lead would result in a significant decrease of Ca2+ influx into the cell. Reduction of Ca2+ entry into the cell will prevent neurotransmitter release and thus impair signalization leading to neurological disease states [62, 63]. Lead can also reduce the expression of brain-derived neurotrophic factor (BDNF), a trans-synaptic signaling molecule that is released from both axons and dendrites which is involved in synaptic development and neurotransmitter release [64]. BDNF activity is also dependent on Ca2+ and thus has been implicated in the development of neurological diseases.
4.3.4 Manganese
Manganese is known to accumulate in the mitochondria of neurons, astrocytes and oligodendrocytes cells and disrupts ATP synthesis [65] by inhibiting the F1/F0 ATP synthase [65] or complex 1 (NADH dehydrogenase) of the mitochondrial respiration chain [66]. More so, it has recently been shown that manganese inhibits ATP synthesis at two sites in the brain mitochondria which are either the glutamate/aspartate exchanger or the complex II (succinate dehydrogenase) depending on the mitochondrial energy source [67]. The disruption of ATP synthesis by manganese leads to decreased intracellular ATP levels and generation of free radicals thereby increasing oxidative stress [68] which may contribute to manganese cellular toxicity [69]. Furthermore, manganese can oxidize dopamine (DA) to react with quinone species thereby disrupting the dopaminergic system (for review, see [70]). This has been shown in animal studies were manganese exposure has led to specific deficits in the dopaminergic system [71]. The DA reactive species are taken up by the dopamine transporter (DAT1) thus causing dopaminergic neurotoxicity [72].
4.4 Biochemical mechanism of heavy metal toxicity
When heavy metals are ingested through food or water into the body, they are acidified by the acid medium of the stomach. In this acidic medium, they are oxidized to their various oxidative states (Zn2+, Cd2+, Pb2+, As2+, As3+, Ag+, Hg2+, etc.) which can readily bind to biological molecules such as proteins and enzymes to form stable and strong bonds. The most common functional group that heavy metals bind is the thio groups (SH group of cysteine and SCH3 group of methionine). Cadmium has been shown to inhibit human thiol transferases such as thioredoxin reductase, glutathione reductase, thioredoxin
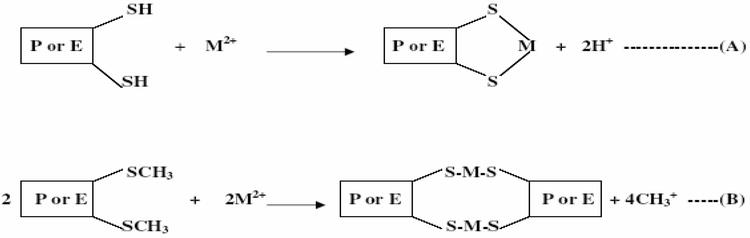
Figure 2.
Reactions of Heavy metals with sulphydryl groups of proteins or enzymes (A) = Intramolecular bonding; (B) = Intermolecular bonding; P = Protein; E = Enzyme; M = Metal.
In the above reaction, the oxidized heavy metal replaces the hydrogen of the SH group and the methyl of the SCH3 group thereby inhibiting the function of the protein or activity of the enzyme. For example, methylmercury (MeHg) strongly inhibits the activity of l-glutamine d-fructose-6-phosphate amidotransferase in yeast [75].
Heavy metal-bound proteins may be a substrate for certain enzymes. In such situations, the heavy metal-bound protein fits into an enzyme in a highly specific pattern to form an enzyme-substrate complex and thus cannot accommodate any other substrate until it is freed. As such, the product of the substrate is not formed as the enzyme is blocked and therefore, the heavy metal remains embedded in the tissue leading to dysfunctions, abnormalities and damages in the body. Inhibition of thiol transferases lead to increased oxidative stress and cell damage. For example, toxic arsenic present in fungicides, herbicides and insecticides can attack –SH groups in enzymes to inhibit their catalytic activities as shown in Figure 3.

Figure 3.
Reaction of arsenic with the thio group of enzymes.
Also, heavy metal toxicity may be induced by the replacement of a metallo-enzyme by another metal ion of similar size. Cadmium displaces zinc and calcium ions from zinc finger proteins and metalloproteins [76, 77]. For instance, cadmium can replace zinc in certain dehydrogenating enzymes, leading to cadmium toxicity. Such replacement can convert the enzyme structurally to an inactive form and completely alter its activity. These heavy metals in their ionic species such as Pb2+, Cd2+, Ag+ Hg2+ and As3+ form very stable biotoxic compounds with proteins and enzymes and are difficult to be dissociated.
Heavy metals may also inhibit protein folding. This was first observed when heavy metals such as cadmium, lead, mercury and arsenite were shown to effectively interfere with the refolding of chemically denatured proteins [78]. It was also observed that when protein misfolded in the presence of heavy metals, the misfolded protein could not be rescued in the presence of reduced glutathione or EDTA chelator. The order of heavy metal in terms of their efficacy in folding inhibition is mercury > cadmium > lead and correlates with the relative stability of their monodentate complexes with imidazole, thiol and carboxylate groups in proteins [79].
Heavy metal may cause proteins to aggregate as arsenite-induced protein aggregation was observed and shown to be concentration-dependent. Also, the aggregates contained a wide variety of proteins enriched in functions related to metabolism, protein folding, protein synthesis and stabilization [79].

Figure 4.
Mechanisms of heavy metal intoxication in humans.
5. Health effects of heavy metal toxicity in humans
Heavy metal toxicity can have several health effects in the body. Heavy metals can damage and alter the functioning of organs such as the brain, kidney, lungs, liver, and blood. Heavy metal toxicity can either be acute or chronic effects. Long-term exposure of the body to heavy metal can progressively lead to muscular, physical and neurological degenerative processes that are similar to diseases such as Parkinson’s disease, multiple sclerosis, muscular dystrophy and Alzheimer’s disease. Also, chronic long-term exposure of some heavy metals may cause cancer [7]. The various health effects of some heavy metals will be highlighted below.
5.1 Arsenic
Arsenic exposure can lead to either acute or chronic toxicity. Acute arsenic poisoning can lead to the destruction of blood vessels, gastrointestinal tissue and can affect the heart and brain. Chronic arsenic toxicity which is termed arsenicosis usually focus on skin manifestations such as pigmentation and keratosis [81]. Lower level exposure to arsenic can cause nausea and vomiting, reduced production of erythrocytes and leukocytes and damage blood vessels, cause abnormal heart beat and pricking sensation in hands and legs. Long-term exposure can lead to the formation of skin lesions, pulmonary disease, neurological problems, peripheral vascular disease, diabetes mellitus, hypertension and cardiovascular disease [82]. Chronic arsenicosis may results to irreversible changes in the vital organs and possibly lead to death. Also, chronic arsenic exposure can promote the development of a number of cancers which include skin cancer, cancers of the bladder, lung, liver (angiosarcoma), and possibly the colon and kidney cancers [82]. Recently in the United States, the tolerable amount of arsenic in drinking water is 50 μg/liter but there is much concern of lowering this standard dose of population exposures to arsenic as the present dose is believed to increase the risk for cancer. Most environmental scientists studying this problem are of the view that the current tolerable limit of arsenic in drinking water or food be reduced.
5.2 Lead
Toxicity due to lead exposure is called lead poisoning. Lead poisoning is mostly related to the gastrointestinal tract and central nervous system in children and adults [83]. Lead poisoning can be either acute or chronic. Acute exposure of lead can cause headache, loss of appetite, abdominal pain, fatigue, sleeplessness, hallucinations, vertigo, renal dysfunction, hypertension and arthritis while chronic exposure can result in birth defects, mental retardation, autism, psychosis, allergies, paralysis, weight loss, dyslexia, hyperactivity, muscular weakness, kidney damage, brain damage, coma and may even cause death [81]. Although lead poisoning is preventable, it still remains a dangerous disease as it can affect most of the organs of the body. Exposure to elevated levels of lead can cause the plasma membrane of the blood brain barrier to move into the interstitial spaces leading to edema [84]. Also, lead exposure can disrupt the intracellular second messenger systems and alter the functioning of the central nervous system. Developing fetuses and children are most vulnerable to neurotoxic effects due to lead exposure. A number of prospective epidemiologic studies in children less than 5 years of age have shown that low-level of lead exposure (5–25 μg/dL in blood) resulted to the impairment of intellectual development which was manifested by the lost of intelligence quotient points [85]. As such, the Centers for Disease Control (CDC) in the United States has reduced the tolerable amount of lead in children’s blood from 25 to 10 μg/dL and recommended universal screening of blood lead for all children.
5.3 Mercury
Mercury is an element that can easily combine with other elements to form inorganic and organic mercury. Exposure to elevated levels of metallic, inorganic and organic mercury can damage the kidney, brain and developing fetus [86] while methyl mercury is highly carcinogenic. Organic mercury is lipophilic in nature and thus can easily penetrate cell membranes. Mercury and its compound affects the nervous system and thus increased exposure of mercury can alter brain functions and lead to tremors, shyness, irritability, memory problems and changes in hearing or vision. Short-term exposure to metallic mercury vapors at higher levels can lead to vomiting, nausea, skin rashes, diarrhea, lung damage, high blood pressure, etc. while short-term exposure to organic mercury poisoning can lead to depression, tremors, headache, fatigue, memory problems, hair loss, etc. Since these symptoms are also common in other illness or disease conditions, diagnosis of mercury poisoning may be difficult in such cases [81]. Chronic levels of mercury exposure can lead to erethism, a disease condition characterized by excitability, tremor of the hands, memory loss, timidity, and insomnia. Also, occupational exposure to mercury as observed by researchers has been associated with measurable declines in performance on neurobehavioral tests of motor speed, visual scanning, visuomotor coordination, verbal and visual memory. Dimethylmercury is a very toxic compound that can penetrate the skin through latex gloves and its exposure at very low dose can cause the degeneration of the central nervous system and death. Mercury exposure to pregnant women can affect the fetus and offspring may suffer from mental retardation, cerebellar symptoms, retention of primitive reflexes, malformation and other abnormalities [87]. This has been confirmed in recent studies in which pregnant women exposed to mercury through dietary intake of whale meat and fish showed reduce motor neuron function, loss of memory, impaired speech and neural transmission in their offspring.
5.4 Cadmium
Cadmium and its compounds have several health effects in humans. The health effects of cadmium exposure are exacerbated due to the inability of the human body to excrete cadmium. In fact, cadmium is re-absorbed by the kidney thereby limiting its excretion. Short-term exposure to inhalation of cadmium can cause severe damages to the lungs and respiratory irritation while its ingestion in higher dose can cause stomach irritation resulting to vomiting and diarrhea. Long-term exposure to cadmium leads to its deposition in bones and lungs. As such, cadmium exposure can cause bone and lung damage [88]. Cadmium can cause bone mineralization as studies on animals and humans have revealed osteoporosis (skeletal damage) due to cadmium. It has been observed that “Itai-itai” disease, an epidemic of bone fractures in Japan is due to cadmium contamination [89]. Increased cadmium toxicity in this population was found to be associated with increased risk of bone fractures in women, as well as decreased bone density and height loss in males and females. Cadmium is highly toxic to the kidney and it accumulates in the proximal tubular cells in higher concentrations. Thus, cadmium exposure can cause renal dysfunction and kidney disease. Also, cadmium exposure can cause disturbances in calcium metabolism, formation of renal stones and hypercalciuria. Cadmium is also classified as group 1 carcinogens for humans by the International Agency for Research on Cancer. Tobacco is the main source of cadmium uptake in smokers and thus, smokers are more susceptible to cadmium intoxication than non-smokers [90]. Also, cadmium can cause testicular degeneration and a potential risk factor for prostate cancer.
5.5 Chromium
Chromium, in its hexavalent form, is the most toxic species of chromium though some other species such as Chromium (III) compounds are much less toxic and cause little or no health problems. Chromium (VI) has the tendency to be corrosive and also to cause allergic reactions to the body. Therefore, breathing high levels of chromium (VI) can cause irritation to the lining of the nose and nose ulcers. It can also cause anemia, irritations and ulcers in the small intestine and stomach, damage sperm and male reproductive system. The allergic reactions due to chromium include severe redness and swelling of the skin. Exposure of extremely high doses of chromium (VI) compounds to humans can result in severe cardiovascular, respiratory, hematological, gastrointestinal, renal, hepatic, and neurological effects and possibly death [91]. Exposure to chromium compounds can result in the formation of ulcers such as nasal septum ulcer which are very common in chromate workers. Exposure to higher amounts of chromium compounds in humans can lead to the inhibition of erythrocyte glutathione reductase, which in turn lowers the capacity to reduce methemoglobin to hemoglobin.
5.6 Iron
Iron salts such as iron sulfate, iron sulfate heptahydrate and iron sulfate monohydrate are of low acute toxicity when exposure is through dermal, oral and inhalation routes. However, other forms of iron are of serious health problems. Iron toxicity occurs in four stages. The first stage which commences 6 h after iron overdose is marked by gastrointestinal effects such as vomiting, diarrhea and gastro-intestinal bleeding. The progression to the second stage occurs 6–24 h after an overdose and it is considered as a latent period of apparent medical recovery. The third stage commences between 12 and 96 h after the onset of clinical symptoms and is characterized by hypotension, shocks, lethargy, hepatic necrosis, tachycardia, metabolic acidosis and may sometimes lead to death [93]. The fourth and final stage usually occurs within 2–6 weeks of iron overdose. This stage is marked by the development of strictures and formation of gastrointestinal ulcerations. Meat is rich in iron and thus meat eating countries are at risk of cancer as excess iron uptake increases the risk of cancer. Asbestos contains about 30% of iron and thus workers who are highly exposed to asbestos are at high risk of asbestosis, a condition which is known to cause lung cancer. Iron is known to generate free radicals which are suggested to be responsible for asbestos related cancer. Iron-induced free radicals can initiate cancer by the oxidation of DNA leading to DNA damage [94].
5.7 Manganese
Although manganese is an essential metal for the body, it recently became a metal of global concern when methylcyclopentadienyl manganese tricarbonyl (MMT), which was known to be toxic was introduced as a gasoline additive. MMT has been claimed to be an occupational manganese hazard and linked with the development of Parkinson’s disease-like syndrome of tremour, gait disorder, postural instability, and cognitive disorder [95]. Exposure to elevated levels of manganese can result in neurotoxicity. Manganism is a neurological disease due to manganese characterized by rigidity, action tremour, a mask-like expression, gait disturbances, bradykinesia, micrographia, memory and cognitive dysfunction, and mood disorder [96]. The symptoms of manganism are very similar to that of Parkinson disease. However, the main differences between manganism and Parkinson disease is the insensitivity of manganism to levodopa (l-DOPA) administration and also the differences in the symptoms and progression of the disease [97].
6. Conclusion
The exposure of heavy metals to humans involve various diverse forms through food and water consumption, inhalation of polluted air, skin contact and most important by occupational exposure at workplace. Though some heavy metals such as iron and manganese are essential for certain biochemical and physiological activities in the body, elevated level in the body can have delirious health effects. Most of the other heavy metals are generally toxic to the body at very low level. The main mechanism of heavy metal toxicity include the generation of free radicals to cause oxidative stress, damage of biological molecules such as enzymes, proteins, lipids, and nucleic acids, damage of DNA which is key to carcinogenesis as well as neurotoxicity. Some of the heavy metal toxicity could be acute while others could be chronic after long-term exposure which may lead to the damage of several organs in the body such as the brain, lungs, liver, and kidney causing diseases in the body.
References
- 1.
Duffus JH. Heavy metals—A meaningless term? Pure and Applied Chemistry. 2002; 74 (5):793-807 - 2.
Li F, Qiu ZZ, Zhang JD. Investigation, pollution mapping and simulative leakage health risk assessment for heavy metals and metalloids in groundwater from a typical brownfield, middle China. International Journal of Environmental Research and Public Health. 2017; 14 (7):768. DOI: 10.3390/ijerph14070768 - 3.
Bradl H, editor. Heavy Metals in the Environment: Origin, Interaction and Remediation. Vol. 6. London: Academic Press; 2002 - 4.
WHO/FAO/IAEA. Trace Elements in Human Nutrition and Health. Switzerland: Geneva: World Health Organization; 1996 - 5.
Florea A-M, Dopp E, Obe G, Rettenmeier AW. Genotoxicity of organometallic species. In: Hirner AV, Emons H, editors. Organic Metal and Metalloid Species in the Environment: Analysis, Distribution, Processes and Toxicological Evaluation. Heidelberg: Springer-Verlag; 2004. pp. 205-219 - 6.
Monisha J, Tenzin T, Naresh A, Blessy BM, Krishnamurthy NB. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology. 2014; 7 (2):60-72 - 7.
Jarup L. Hazards of heavy metal contamination. British Medical Bulletin. 2003; 68 (1):167-182 - 8.
Krishna AK, Mohan KR. Distribution, correlation, ecological and health risk assessment of heavy metal contamination in surface soils around an industrial area, Hyderabad, India. Environment and Earth Science. 2016; 75 :411. DOI: 10.1007/s12665-015-5151-7 - 9.
Hu H. Human health and heavy metals exposure. In: McCally M, editor. Life Support: The Environment and Human Health. Massachusetts, USA: MIT Press; 2002 - 10.
Sauvé S. Time to revisit arsenic regulations: Comparing drinking water and rice. BMC Public Health. 2014; 14 :465 - 11.
Thurmer K, Williams E, Reutt-Robey J. Autocatalytic oxidation of lead crystallite surfaces. Science. 2002; 297 (5589):2033-2035 - 12.
Wani AL, Ara A, Usmani JA. Lead toxicity: A review. Interdisciplinary Toxicology. 2015; 8 (2):55-64 - 13.
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia A. Cadmium toxicity and treatment: An update. Caspian Journal of Internal Medicine. 2017; 8 (3):135-145 - 14.
Unaegbu M, Engwa GA, Abaa QD, Aliozo SO, Ayuk EL, Osuji GA, et al. Heavy metal, nutrient and antioxidant status of selected fruit samples sold in Enugu, Nigeria. International Journal of Food Contamination. 2016; 3 (7):1-8 - 15.
Engwa AG, Ihekwoaba CJ, Ilo US, Unaegbu M, Ayuk LE, Osuji AG. Determination of some soft drink constituents and contamination by some heavy metals in Nigeria. Toxicology Reports. 2015; 2 :384-390 - 16.
Ghani A. Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egyptian Acadmic Journal of Biological Sciences. 2011;2 (1):9-15 - 17.
Zayed AM, Terry N. Chromium in the environment: Factors affecting biological remediation. Plant and Soil. 2003; 249 (1):139-156 - 18.
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Copper. Atlanta: U.S. Department of Health and Humans Services, Public Health Service, Centers for Diseases Control; 2004 - 19.
Ferner DJ. Toxicity, heavy metals. eMedical Journal. 2001; 2 (5):1-8 - 20.
Ming-Ho Y. Environmental Toxicology: Biological and Health Effects of Pollutants, Chap.12. 2nd ed. Boca Raton, USA: CRC Press LLC; 2005 ISBN 1-56670-670-2 - 21.
Florea A-M, Busselberg D. Occurrence, use and potential toxic effects of metals and metal compounds. Biometals. 2006; 19 :419-427 - 22.
Valko M, Morris H, MTD C. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005; 12 :1161-1208 - 23.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Molecular and Cellular Biochemistry. 2004; 266 :37-56 - 24.
Bucher JR, Tien M, Aust SD. The requirement for ferric in the initiation of lipid peroxidation by chelated ferrous iron. Biochemistry and Biophysical Research Communication. 1983; 111 :777-784 - 25.
Eaton JW, Qian MW. Molecular bases of cellular iron toxicity. Free Radical Biology Medicine. 2002; 32 :833-840 - 26.
Lloyd RV, Hanna PM, Mason RP. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radical Biology and Medicine. 1997; 22 :885-888 - 27.
Brezova V, Valko M, Breza M, Morris H, Telser J, Dvoranova D, et al. Role of radicals and singlet oxygen in photoactivated DNA cleavage by the anticancer drug camptothecin: An electron paramagnetic resonance study. Physical Chemistry B. 2003; 107 :2415-2425 - 28.
Burkitt MJ. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: Roles of lipid hydroperoxides, α-tocopherol, thiols, and ceruloplasmin. Archive of Biochemistry and Biophysics. 2001; 394 :117-135 - 29.
Liu KJ, Shi XL. In vivo reduction of chromium (VI) and its related free radical generation. Molecular and Cellular Biochemistry. 2001; 222 :41-47 - 30.
Hanna PM, Kadiiska MB, Mason RP. Oxygen-derived free-radical and active oxygen complex-formation from cobalt (II) chelates in vitro. Chemistry Research Toxicology. 1992; 5 :109-115 - 31.
Crans DC, Smee JJ, Gaidamauskas E, Yang LQ. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chemistry Review. 2004; 104 :849-902 - 32.
Pi J, Horiguchi S, Sun Y, Nikaido M, Shimojo N, Hayashi T, et al. A potential mechanism for the impairment of nitric oxide formation caused by prolonged oral exposure to arsenate in rabbits. Free Radical Biology and Medicine. 2003; 35 :102-113 - 33.
Rin K, Kawaguchi K, Yamanaka K, Tezuka M, Oku N, Okada S. DNA-strand breaks induced by dimethylarsinic acid, a metabolite of inorganic arsenics, are strongly enhanced by superoxide anion radicals. Biology and Pharmacology Bulletin. 1995; 18 :45-48 - 34.
Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. Journal of Cancer Prevention. 2015; 20 :232-240 - 35.
Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harbor Perspectives in Medicine. 2016; 6 (1-6):a026104 - 36.
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. Journal of Toxicology. 2011; 2011 :1-13 - 37.
Bjørklund G, Aaseth J, Chirumbolo S, Urbina MA, Uddin R. Efects of arsenic toxicity beyond epigenetic modiications. Environmental Geochemistry and Health. 2017; 39 :1-11 - 38.
Park YH, Kim D, Dai J, Zhang Z. Human bronchial epithelial BEAS-2B cells, an appropriate in vitro model to study heavy metals induced carcinogenesis. Toxicology and Applied Pharmacology. 2015; 287 (3):240-245 - 39.
Saleha Banu B, Danadevi K, Jamil K, Ahuja YR, Visweswara Rao K, Ishaq M. In vivo genotoxic effect of arsenic trioxide in mice using comet assay. Toxicology. 2001; 162 :171-177 - 40.
Hartwig A, Schwerdtle T. Interactions by carcinogenic metal compounds with DNA repair processes: Toxicological implications. Toxicology Letter. 2002; 127 :47-54 - 41.
García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E. Arsenic exposure and cancer mortality in a US-based prospective cohort: The strong heart study. Cancer Epidemiology, Biomarkers & Prevention. 2013; 22 :1944-1953 - 42.
Silbergeld EK, Waalkes M, Rice JM. Lead as a carcinogen: Experimental evidence and mechanisms of action. American Journal of Industrial Medicine. 2000; 38 (3):316-323 - 43.
Crespo-Lopez ME, Macedo GL, Pereira SI, Arrifano GP, Picanco-Diniz DL, do Nascimento JL, et al. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacological Research. 2009; 60 (4):212-220 - 44.
Zhou C, Huang C, Wang J, Huang H, Li J, Xie Q, et al. LncRNA MEG3 downregulation mediated by DNMT3b contributes to nickel malignant transformation of human bronchial epithelial cells via modulating PHLPP1 transcription and HIF-1α translation. Oncogene. 2017; 36 :3878-3889 - 45.
Zambelli B, Uversky VN, Ciurli S. Nickel impact on human health: An intrinsic disorder perspective. Biochimica Et Biophysica Acta (BBA) – Proteins and Proteomics. 2016; 1864 (12):1714-1731 - 46.
Neal AP, Guilarte TR. Mechanisms of heavy metal neurotoxicity: Lead and manganese. Journal of Drug Metabolism and Toxicology. 2012; S5 :002 - 47.
Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl- d -aspartate receptor subunit changes are associated with lead induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99 :233-242 - 48.
Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982; 297 :681-683 - 49.
Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996; 87 :1327-1338 - 50.
Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Letters. 1990; 261 :124-130 - 51.
Neal AP, Worley PF, Guilarte TR. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology. 2011; 32 :281-289 - 52.
Guilarte TR, Miceli RC, Jett DA. Neurochemical aspects of hippocampal and cortical Pb2+ neurotoxicity. Neurotoxicology. 1994; 15 :459-466 - 53.
Neal AP, Guilarte TR. Molecular neurobiology of lead (Pb(2+)): Effects on synaptic function. Molecular Neurobiology. 2010; 42 :151-160 - 54.
Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000; 25 :685-694 - 55.
Xu SZ, Rajanna B. Glutamic acid reverses Pb2+−induced reductions of NMDA receptor subunits in vitro. Neurotoxicology. 2006; 27 :169-175 - 56.
Guilarte TR, McGlothan JL, Nihei MK. Hippocampal expression of N-methyl- d -aspartate receptor (NMDAR1) subunit splice variant mRNA is altered by developmental exposure to Pb2+. Molecular Brain Research. 2000;76 :299-305 - 57.
Guilarte TR, McGlothan JL. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+−exposed rats: Implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Research and Molecular Brain Research. 2003; 113 :37-43 - 58.
Lasley SM, Gilbert ME. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain Research. 1996; 736 :125-134 - 59.
Xiao C, Gu Y, Zhou CY, Wang L, Zhang MM. Pb2+ impairs GABAergic synaptic transmission in rat hippocampal slices: A possible involvement of presynaptic calcium channels. Brain Research. 2006; 1088 :93-100 - 60.
Braga MF, Pereira EF, Albuquerque EX. Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Research. 1999; 826 :22-34 - 61.
Peng S, Hajela RK, Atchison WD. Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells. Molecular Pharmacology. 2002; 62 :1418-1430 - 62.
Waites CL, Garner CC. Presynaptic function in health and disease. Trends in Neuroscience. 2011; 34 :326-337 - 63.
Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: Schizophrenia as a disease of the synapse. Trends in Neuroscience. 2001; 24 :479-486 - 64.
Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005; 46 :401-405 - 65.
Milatovic D, Gupta RC, Yin Z, Zaja-Milatovic S, Aschner M. Manganese in Reproductive and Developmental Toxicology. 2017. pp. 567-581. DOI: 10.1016/B978-0-12-804239-7.00032-9 - 66.
Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): Special reference to mitochondrial [Fe-S] containing enzymes. Toxicology and Applied Pharmacology. 2001; 175 :160-168 - 67.
Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicology Applied Pharmacology. 2010; 249 :65-75 - 68.
Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicology and Applied Pharmacology. 2009; 240 :219-225 - 69.
Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: A search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006; 27 :765-776 - 70.
Paris I, Segura-Aguilar J. The role of metal ions in dopaminergic neuron degernation in parkinsonism and Parkinson’s disease. Monatsh Chemistry. 2011; 142 :365-374 - 71.
Burton NC, Guilarte TR. Manganese neurotoxicity: Lessons learned from longitudinal studies in nonhuman primates. Environmental Health Perspectives. 2009; 117 :325-332211 - 72.
Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans . PLoS Genetics. 2010;6 :e1001084 - 73.
Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. The Journal of Biological Chemistry. 2000; 275 :26556-26565 - 74.
Duruibe JO, Ogwuegbu MO, Egwurugwu JN. Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences. 2007; 2 (5):112-118 - 75.
Naganuma A, Miura N, Kaneko S, Mishina T, Hosoya S, Miyairi S, et al. GFAT as a target molecule of methylmercury toxicity in Saccharomyces cerevisiae . FASEB Journal. 2000;14 :968-972 - 76.
Hartwig A. Zinc finger proteins as potential targets for toxic metal ions: Differential effects on structure and function. Antioxidant Redox Signal. 2001; 3 :625-634 - 77.
Faller P, Kienzler K, Krieger-Liszkay A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of photosystem II by competitive binding to the essential Ca2+ site. Biochemical Biophysics Acta. 2005; 1706 :158-164 - 78.
Sharma SK, Goloubinoff P, Christen P. Heavy metal ions are potent inhibitors of protein folding. Biochemical and Biophysics Research Communication. 2008; 372 :341-345 - 79.
Tamás MJ, Sharma SK, Ibstedt S, Jacobson T, Christen P. Heavy metals and metalloids As a cause for protein misfolding and aggregation. Biomolecules. 2014; 4 :252-267 - 80.
Jacobson T, Navarrete C, Sharma SK, Sideri TC, Ibstedt S, Priya S, et al. Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast. Journal of Cell Science. 2012; 125 :5073-5083 - 81.
Martin S, Griswold W. Human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens. 2009; 15 :1-6 - 82.
Huy TB, Tuyet-Hanh TT, Johnston R, Nguyen-Viet H. Assessing health risk due to exposure to arsenic in drinking water in Hanam Province, Vietnam. International Journal of Environmental Research and Public Health. 2014; 11 :7575-7591 - 83.
Markowitz M. Lead poisoning. Pediatry Review. 2000; 21 (10):327-335 - 84.
Teo J, Goh K, Ahuja A, Ng H, Poon W. Intracranial vascular calcifi cations, glioblastoma multiforme, and lead poisoning. American Journal of Neuroradiology. 1997; 18 :576-579 - 85.
Brown MJ, Margolis S. Lead in drinking water and human blood Lead levels in the United States. In: The Morbidity and Mortality Weekly Report (MMWR). Washington, DC: Center for Disease Control and Prevention (CDC); 2012 - 86.
Alina M, Azrina A, Mohd Yunus AS, Mohd Zakiuddin S, Mohd Izuan Effendi H, Muhammad Rizal R. Heavy metals (mercury, arsenic, cadmium, plumbum) in selected marine fi sh and shellfi sh along the straits of Malacca. International Food Research Journal. 2012; 19 (1):135-140 - 87.
Bernhoft RA. Mercury toxicity and treatment: A review of the literature. International Journal of Environmental Research and Public Health. 2012; 2012 :1-10 - 88.
Bernard A. Cadmium & its adverse effects on human health. Indian Journal Medical Research. 2008; 128 (4):557-564 - 89.
Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Kido T. Causes of death in patients with Itaiitai disease suffering from severe chronic cadmium poisoning: A nested case-control analysis of a follow-up study in Japan. BMJ Open. 2017; 7 :e015694 - 90.
Mudgal V, Madaan N, Mudgal A, Singh RB, Mishra S. Effect of toxic metals on human health. Open Nutraceuticals Journal. 2010; 3 :94-99 - 91.
Shekhawat K, Chatterjee S, Joshi B. Chromium toxicity and its health hazards. International Journal of Advanced Research. 2015; 7 (3):167-172 - 92.
Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA. Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genetics Molecular Biology. 2006;29 (1):148-158 - 93.
Hillman RS. Hematopoietic agents: Growth factors, minerals, and vitamins. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 1487-1518 - 94.
Bhasin G, Kauser H, Athar M. Iron augments stage-I and stage-II tumor promotion in murine skin. Cancer Letter. 2002; 183 (2):113-122 - 95.
O'Neal S, Zheng W. Manganese toxicity upon overexposure: A decade in review. USA. Current Environmental Health Reports. 2015; 2 :315-328 - 96.
Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. European Journal of Neurology. 2006; 13 :1139-1141 - 97.
Guilarte TR. Manganese and Parkinson’s disease: A critical review and new findings. Environmental Health Perspectics. 2010; 118 :1071-1080