Nature and synthetic zinc ionophores.
Abstract
Zinc is a trace metal ion that has a role in both physiological and pathological processes, making it one of the most common and necessary components involved in brain function. Besides, zinc is required for cell proliferation control in a variety of mechanisms, including hormonal regulation of cell division. Also, zinc serves as a biochemical signal to immune cells and transcription factors involved in the synthesis of inflammatory cytokines. On the other hand, zinc has a variety of crucial roles in neurogenesis and also acts as a neuromodulator on a wide range of membrane receptors, ion channels, and transporters. Zinc is produced by neurons under several conditions to activate microglia. The link between zinc dysregulation and psychiatric disorder was that zinc acts as an inhibitory modulator at the N-methyl-D aspartic acid (NMDA) glutamate receptor. Ionophores are ion carrier molecules that reversibly bind and transport ions through biological membranes. Ionophores can be natural or synthetic products. Zinc ionophores such as quercetin, epigallocatechin gallate (EGCG), hinokitol, and proanthocyanidins have been shown to protect brain health, particularly in depression clinically significant depression and depressive symptoms in post-COVID-19 syndrome may have severe implications as it relates to life outcomes quality, herein according to previous research studies, we showed zinc deficiency as a possible risk factor for depression symptoms, which were commonly observed following severe infection of COVID-19.
Keywords
- ionophore
- zinc
- cytokines
- quercetin
- EGCG
- hinokitol
- proanthocyanidins
1. Introduction
Zinc is a trace metal ion that has a role in both physiological and pathological processes, making it one of the most common and necessary components involved in brain function. The cortex, amygdala, olfactory bulb, and hippocampus neurons all carry “free ionic zinc” (Zn2+), which appears to have the largest concentration of zinc in the brain. Zinc is involved in the physiochemical function of enzymes, proteins, and signal transcription factors, as well as the maintenance of numerous homeostatic systems, functioning as structural, regulatory, and catalytic cofactors for enzymes including DNA and RNA polymerases, histone deacetylases, and DNA ligases. Zinc is also required for cell proliferation and genomic integrity [1, 2, 3, 4, 5].
As a neuromodulator, zinc is produced during synaptic transmission and attaches to presynaptic or postsynaptic membrane receptors, allowing it to translocate from presynaptic terminals to postsynaptic neurons [6, 7]. Zinc can be found in glutamatergic neurons’ synaptic vesicles. Zinc is therefore liberated from glutamatergic synaptic vesicles and then interacts with excitatory and inhibitory amino acid receptors (N-methyl-D aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and γ-aminobutyric acid (GABA) [8, 9, 10]. Because of its actions on numerous voltage-gated ion channels, extracellular Zn2+ can modify the excitability of nerve cells [11, 12, 13].
Besides, zinc is required for cell proliferation control in a variety of mechanisms, including hormonal regulation of cell division. Also, zinc serves as a biochemical signal to immune cells and transcription factors involved in the synthesis of inflammatory cytokines. Zinc supplementation has been proven in trials to reduce rates of infection and proinflammatory cytokine secretion. Zinc also possesses metal-binding characteristics and is widely recognized for its antioxidant qualities [14, 15]. Zinc deficiency causes apoptosis in neurons via the mitochondrial pathway [16, 17]. Zinc has just lately been discovered to have a role in intracellular signaling as a second messenger. It is also used by immune cells as a molecular signal. Zinc controls a variety of transcription factors that control gene expression and are engaged in the signal transduction of inflammatory cytokines and adhesion molecules. Zinc helps to preserve genomic stability by regulating redox homeostasis, DNA repair, synthesis, and methylation [18, 19].
2. Role of zinc in the brain
2.1 Role of zinc in neurogenesis and synaptic transmission
Zinc has a variety of crucial roles in neurogenesis [4]. Zinc deficiency decreases the neurogenesis process and impairs the expression of genes involved in hippocampus proliferation and neuronal development in the postnatal rat cerebellum [20]. Further, zinc deficiency reduces the proliferation of the human neuroblastoma cell line, promotes apoptosis, and inhibits retinoic-acid-induced neuronal development in cultured cells [1, 21].
Of note, zinc is found in the presynaptic glutamatergic vesicles across the brain, including the cerebral cortex, limbic system, hippocampus, and olfactory bulb [22].
It acts as a neuromodulator on a wide range of membrane receptors, ion channels, and transporters [23]. Synaptic zinc, in particular, is enhanced via a specialized zinc transporter, ZnT3, and is coreleased with glutamate during action potential-induced exocytosis [24]. These also have an impact on synaptic transmission, which interacts with receptors and channels that regulate auditory processing [25, 26]. Synaptic zinc has been discovered to inhibit NMDA receptors, GABA-A receptors, and calcium channels while activating AMPA and glycine receptors [27, 28, 29, 30]. Zinc also has vital effects on other kinds of receptors, including serotonin, dopamine, and acetylcholine receptors, as well as voltage-gated ion channels for sodium, potassium, calcium, and chlorine [29, 31].
Synaptic zinc regulates sensory processing and improves acuity in the discrimination of different sensory stimuli. Synaptic zinc plasticity leads to prolonged adaptations and sense memories. Recently, the mechanism of this long-term synaptic zinc plasticity has been described as being due to group 1 metabotropic glutamate receptors (G1 mGluRs)-dependent mechanism that triggers a bidirectional long-term change in synaptic zinc signaling [32].
2.2 Role of zinc at depression
No one denies that depression treatment is a gateway to overcoming many social and psychological problems that affect millions of people all over the world. Many factors play a role in depressive-like behaviors, such as impairment of functions of the hippocampus and the prefrontal cortex. These brain parts play an important role in decision-making processes, so any dysfunction at this area can induce a predisposition to negative feelings, and many glucocorticoid receptors are involved in these areas [33].
In terms of both pharmacological and clinical/epidemiological data, recent years have provided additional evidence confirming the role of zinc in depression. Zinc demonstrated antidepressant-like efficacy in preclinical studies and depressive models. Clinical evidence suggested that zinc supplementation might be beneficial in people suffering from depression. Zinc supplementation has been demonstrated to be beneficial as adjuvant therapy or as a stand-alone intervention for depression. Furthermore, zinc consumption has been linked to an increased risk of depression. Dietary zinc restriction was found to be a causal factor in the development of depressive-like symptoms or anhedonia in mouse studies [34]. Some epidemiological studies have reported that reduced nutritional zinc consumption is related to depression in females but not in males [35]. Even though the first prospective study examining the relationship between zinc intake and depression risk found a small but significant inverse correlation between them, a 20-year follow-up study found that a reduced dietary zinc intake protects from depression in men who were not previously depressed. However, because the research participants were all men with a hospital discharge diagnosis of unipolar depression, the findings cannot be applied to women or patients who did not require hospitalization. On the contrary, a reduced nutritional zinc intake was found to be a risk factor for depression in a prospective analysis of both men and women [36]. Mice missing the G-protein-coupled receptor 39 (GPR39), a zinc-activated receptor, show depressive-like behavior [37]. TC-G-1008, a GPR39 agonist, was recently discovered to have antidepressant-like effects [38]. These findings add to the growing body of evidence that zinc is useful in the treatment of depression.
Meta-analyses support the use of zinc as a supplement in the treatment of severe depression, and single research currently supports the use of zinc for psychotic symptoms [39]. Zinc deficiency has also been linked to neuropsychiatric symptoms such as altered behavior and cognition, learning difficulties, and depression [40, 41, 42].
The link between zinc dysregulation and psychiatric disorder was that zinc acts as an inhibitory modulator at the NMDA glutamate receptor [43, 44, 45]. In addition, the inhibitory effects on the nicotinic acetylcholine receptor (nAChR), GSK3 (glycogen synthase kinase 3beta), and NOS (nitric oxide synthase) are also relevant to depressive processes [46, 47].
Numerous studies show lower zinc blood levels in depressed people compared with healthy people, with a meta-analysis showing depressive symptomatology at zinc serum levels of 1.8 M or below [48]. In several investigations, zinc supplementation enhanced mood in those who were suffering from treatment-resistant depression [41, 49].
Zinc’s effect on the brain-derived neurotrophic factor (BDNF), a growth factor that promotes neurogenesis and differentiation, may be connected to depression. The hippocampus is a center of lifelong neurogenesis, and periods of significant depression are associated with reduced BDNF expression and neuro/synaptogenesis. Rodents on a zinc-deficient diet had lower zinc levels in the hippocampus vesicles, a part of the brain where zinc levels are generally greater, as well as lower amounts of progenitor cells and immature neurons. Zinc-rich diets, on the other hand, increased amounts of progenitor cells [3, 41]. The GPR39 receptor is most likely a critical connection in the interaction between zinc and the serotonergic system, which is required for antidepressants that affect the serotonin pathway to work [34].
2.3 Zinc and neuroimmunity
Of note, laboratory animal models showed that zinc insufficiency induces thymus and lymphoid tissue atrophy. It lowers the number of spleen cells and the sensitivity to antigens that are both T-cell-dependent and -independent [50]. Microglia is a kind of immune cell found in the central nervous system (CNS) [51]. The link between zinc and microglial activation reflects an undiscovered process that may play a role in neuropathy. However, zinc is produced by neurons under several conditions to activate microglial [52].
3. Zinc ionophores
Ionophores are ion carrier molecules that reversibly bind and transport ions through biological membranes. Many ionophores are lipid-soluble ion transporters that traverse the cell membrane. Ionophores accelerate ion transport through hydrophobic membranes such as liquid polymeric membranes (carrier-based ion-selective electrodes), lipid bilayers in live cells, or synthetic vesicles (liposomes). A hydrophilic core and a hydrophobic section interact with the membrane in the structure of an ionophore [53]. Many microorganisms, fungi, and plants naturally manufacture ionophores, which import ions into their cells and function as a defense against competing or harmful species. Ionophores made from synthetic materials have also been developed. Ionophores that select for cations and anions have a wide range of uses in the analysis [54]. When paired with the ion they bind, these chemicals have been proven to have a variety of biological effects as well as a synergistic impact [55]. Ionophores change the permeability of biological membranes in the direction of certain ions for which they have affinity and selectivity (Figure 1). An ionophore has a hydrophilic core and a hydrophobic section that interacts with the membrane in terms of structure. An ionophore-ion complex is formed when ions are bound to the hydrophilic center. X-ray crystallography has confirmed the structure of the ionophore-ion complex [58].
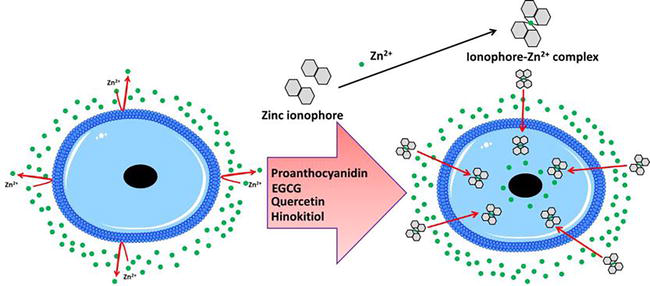
Figure 1.
Zinc ionophores mechanism in penetrating cell membranes. Two ionophore molecules can mediate intracellular zinc accumulation by exchanging extracellular Zn2+ with 2H+ [
Zinc ionophores (Table 1; Figure 2) have been shown to inhibit replication of various viruses in vitro, including coxsackievirus [63, 65], equine arteritis virus [68], coronavirus [68], HCV [69], HSV [70], HCoV-229E [71], HIV [72, 73], mengovirus [63, 65], MERS-CoV [71], rhinovirus [65], SARS-CoV-1 [68], and Zika virus [74].
| Zinc Ionophore | Sources | References |
|---|---|---|
| Calcimycin | [59, 60] | |
| Chloroquine | [61] | |
| Clioquinol | Synthetic ionophore | [55] |
| Diiodohydroxyquinoline | Synthetic ionophore | [62] |
| Dithiocarbamates | Synthetic ionophore | [63] |
| EGCG | [64] | |
| Hinokitiol | [65] | |
| Proanthocyanidins | Grape seed | [66] |
| PBT2 | Synthetic analog of 8-hydroxyquinoline | [67] |
| Pyrithione | [65] | |
| Quercetin | Vegetables, fruits, berries, herbs, trees, and other plants | [64] |
| Zincophorin | [55] |
Table 1.
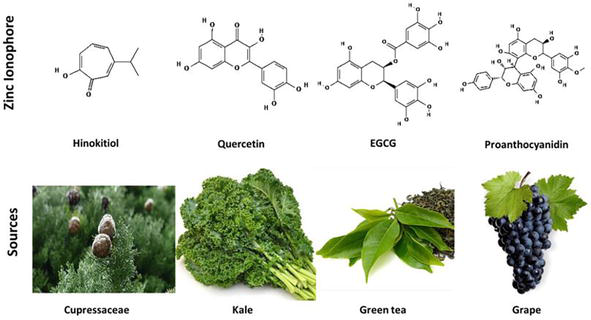
Figure 2.
Natural zinc ionophores and their sources. Chemical structures of ionophores obtained from Pubchem database (Hinokitiol, CID: 3611; quercetin, CID: 5280343; EGCG, CID: 65064; Proanthocyanidin, CID: 108065).
3.1 Examples of zinc ionophores and their role in brain health, depression as an example
3.1.1 Quercetin
Quercetin has attracted the attention of many researchers because of its capacity to pass the blood–brain barrier. It appears in the brain after hours of administration and plays a key function in the central nervous system [75]. Discoveries from animal model research reported that antioxidant, anti-inflammatory, and neuroprotective effects of quercetin keep neurons in healthy condition by inhibiting the formation of hydroperoxide, reducing free radicals, and restoring antioxidant enzymes. Further, the study of quercetin at rat models proves its antidepressant action [76, 77]. Also, quercetin can reduce stress and depressive-like symptoms [75].
3.1.2 Epigallocatechin gallate (EGCG)
EGCG may act as a new antidepressant by inhibiting neuroinflammation, which may help to alleviate depression. Models of chronic unexpected mild stress (CUMS) in rats have been created in experimental investigations of depression [78]. Although the etiology of depression is not well understood, one popular theory is that depressed people have greater amounts of cytokines such as IL-6 due to lower levels of amines such as serotonin, noradrenaline, and dopamine [79]. EGCG injection improved depressed behavior in rats by reducing Il-6 levels in the hippocampus. As a result, EGCG was suggested to be used as a new antidepressant to reduce neuroinflammation, which could help to alleviate depression [80].
3.1.3 Hinokitol
Hinokitiol (β-thujaplicin) is a monoterpenoid that occurs naturally in the wood of Cupressaceae plants. It is a natural zinc ionophore that is safe to use. Because of its powerful, broad-spectrum antiviral, antibacterial, antifungal, anti-inflammatory, and anticancer effects, it is frequently employed in oral care and medicinal products. It is also a food additive that does not build up in the body. Throughout years of use, there have been no reports of allergic, poisonous, or adverse consequences in the literature. Hinokitiol is a safe zinc ionophore that increases the intracellular pool of labile zinc by facilitating zinc influx into cells [81].
3.1.4 Proanthocyanidins
Proanthocyanidins (GSPs), which comprise dimers, trimers, oligomers, and oligomers of catechin and epicatechin, are known to have antidepressant properties. Recent research has demonstrated the mechanism of GSPs’ antidepressant effects in female juvenile prenatally stressed offspring rats. The main pathway was that GSPs work synergistically to inhibit oxidative stress and inflammatory response activator proteins [66].
4. Cross talk between zinc deficiency and depression caused by COVID-19
High rates of neuropsychiatric symptoms (e.g., depression) have been observed among patients affected by COVID-19, suggesting an effect of COVID-19 on the human central nervous system (CNS) [82, 83, 84, 85]. It was showed globally that depression is a leading cause of disability [86]. Accordingly, clinically significant depression and depressive symptoms in post-COVID-19 syndrome may have severe implications as it relates to life outcomes quality [86]. Herein according to previous research studies, we showed zinc deficiency as a possible risk factor for depression symptoms, which were commonly observed following severe infection of COVID-19. A meta-analysis of 17 observational studies found that blood Zn2+ concentrations were lower in depressed subjects than in control subjects [48]. Interestingly, a recent study showed that a significant number of patients with COVID-19 were zinc-deficient [87], and a higher number of zinc-deficient COVID-19 patients had prolonged hospital stay when compared with those with normal zinc levels and required intensive care unit (ICU) [87]. A significant positive correlation was observed between the prevalence of zinc deficiency and COVID-19 cases [88]. A pooled analysis of 1532 COVID-19 patients suggested that zinc deficiency was associated with a sixfold increased risk of severe disease and 16-fold increased risk of death via elevating LDH [89]. The elevated LDH in the present study was probably indicative of severe disease [87]. Because zinc has a critical role in regulating functions of the human brain, many disorders have been linked with Zn2+ deficiency, including neurological diseases, such as psychiatric disorders, (depression) [48, 89] and schizophrenia [90]. Consequently, the clinical picture, which is common in severe COVID-19 patients and is referred to as “Depression” [82, 83, 84, 85], is nothing more than depression seen in zinc deficiency [48, 87, 88, 89]. Most likely, depression and other mental problems in these patients also develop due to zinc deficiency in nerve cells in the brain.
The first study revealing a relationship between depression and dietary zinc deficiency was conducted by Amani et al. [90]. This study included 23 young females diagnosed with moderate and severe depression and 23 healthy volunteers who were age-matched. The findings revealed that the depressive group’s daily zinc consumption and serum zinc concentration were both lower than the healthy women’s. Moreover, an inverse correlation between serum zinc concentration and the depression scores was obtained [90]. According to the World Health Organization (WHO), zinc deficiency affects at least one-third of the world’s population [91]. The fact that zinc deficiency is linked to the risk of infection and severe advancement of COVID-19 [91] gives a first significant clue on a link between zinc deficiency and the risk of infection as well as its symptoms with unknown etiology such as depression and suggests possible benefits of zinc supplementation. Owing to Zn2+ neuroprotective properties, it is not surprising that Zn2+ supplementation could be effective not only on COVID-19-related symptoms but also on virus replication, as well as on COVID-19-related inflammation and neurological damage [92]. In vitro, Zn2+ inhibits Coronavirus and Arter virus RNA polymerase activity, and zinc ionophores prevent these viruses from replicating in cell culture [93]. Zinc ionophore may play a role in therapeutic management for COVID-19 [94].
5. Conclusions
Zinc deficiency has been linked to different nervous system disorders. Because zinc is not fat-soluble, it requires transporters called zinc ionophores, which facilitate the entrance of zinc in cytoplasm increasing its level of concentration in the body after consumption. The role of zinc in protecting brain cells has been extensively studied recently particularly in depression treatment. Therefore, natural zinc ionophores plus zinc supplements, which are commercially available, could be a new way to treatment of many neuropsychiatric disorders. Zinc ionophore may play a role in therapeutic management for COVID-19 and postcovid-19 depression.
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BDNF | brain-derived neurotrophic factor |
| CUMS | chronic unexpected mild stress |
| EGCG | Epigallocatechin gallate |
| G1 mGluRs | group 1 metabotropic glutamate receptors |
| GABA | γ-aminobutyric acid, gamma-Aminobutyric acid |
| GPR39 | G-protein coupled receptor 39 |
| GSK3 | glycogen synthase kinase 3beta |
| GSPs | Proanthocyanidins |
| nAChR | nicotinic acetylcholine receptor |
| NMDA | N-methyl-D aspartic acid |
| NOS | nitric oxide synthase |
| LDH | Lactate dehydrogenase |
References
- 1.
Adamo AM, Zago MP, Mackenzie GG, et al. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotoxicity Research. 2010; 17 :1-14. DOI: 10.1007/s12640-009-9067-4 - 2.
Gao H-L, Zheng W, Xin N, et al. Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotoxicity Research. 2009; 16 :416-425. DOI: 10.1007/s12640-009-9072-7 - 3.
Suh SW, Won SJ, Hamby AM, et al. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. Journal of Cerebral Blood Flow and Metabolism. 2009; 29 :1579-1588. DOI: 10.1038/jcbfm.2009.80 - 4.
Corniola RS, Tassabehji NM, Hare J, et al. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Research. 2008; 1237 :52-61. DOI: 10.1016/j.brainres.2008.08.040 - 5.
Moon M-Y, Kim HJ, Choi BY, et al. Zinc promotes adipose-derived mesenchymal stem cell proliferation and differentiation towards a neuronal fate. Stem Cells International. 2018; 2018 :5736535. DOI: 10.1155/2018/5736535 - 6.
Jan H-H, Chen I-T, Tsai Y-Y, Chang Y-C. Structural role of zinc ions bound to postsynaptic densities. Journal of Neurochemistry. 2002; 83 :525-534. DOI: 10.1046/j.1471-4159.2002.01093.x - 7.
Grabrucker AM, Knight MJ, Proepper C, et al. Concerted action of zinc and ProSAP/shank in synaptogenesis and synapse maturation. The EMBO Journal. 2011; 30 :569-581. DOI: 10.1038/emboj.2010.336 - 8.
Draguhn A, Verdorn TA, Ewert M, et al. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990; 5 :781-788. DOI: 10.1016/0896-6273(90)90337-f - 9.
Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987; 328 :640-643. DOI: 10.1038/328640a0 - 10.
Tabata T, Ishida AT. A zinc-dependent Cl− current in neuronal somata. The Journal of Neuroscience. 1999; 19 :5195-5204. DOI: 10.1523/JNEUROSCI.19-13-05195.1999 - 11.
Koh JY, Suh SW, Gwag BJ, et al. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996; 272 :1013-1016. DOI: 10.1126/science.272.5264.1013 - 12.
Weiss JH, Hartley DM, Koh JY, Choi DW. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993; 10 :43-49. DOI: 10.1016/0896-6273(93)90240-r - 13.
Sensi SL, Yin HZ, Carriedo SG, et al. Preferential Zn2+ influx through Ca2+−permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proceedings of the National Academy of Sciences of the United States of America. 1999; 96 :2414-2419. DOI: 10.1073/pnas.96.5.2414 - 14.
MacDonald RS. The role of zinc in growth and cell proliferation. The Journal of Nutrition. 2000; 130 :1500S-1508S. DOI: 10.1093/jn/130.5.1500S - 15.
Jarosz M, Olbert M, Wyszogrodzka G, et al. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017; 25 :11-24. DOI: 10.1007/s10787-017-0309-4 - 16.
Choi DW, Koh JY. Zinc and brain injury. Annual Review of Neuroscience. 1998; 21 :347-375. DOI: 10.1146/annurev.neuro.21.1.347 - 17.
Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nature Reviews. Neuroscience. 2009; 10 :780-791. DOI: 10.1038/nrn2734 - 18.
Prasad AS. Zinc in human health: Effect of zinc on immune cells. Molecular Medicine. 2008; 14 :353-357. DOI: 10.2119/2008-00033.Prasad - 19.
Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017; 9 :1286, 2. DOI: 10.3390/nu9121286 - 20.
Dvergsten CL, Fosmire GJ, Ollerich DA, Sandstead HH. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. II. Impaired maturation of Purkinje cells. Brain Research. 1984; 318 :11-20. DOI: 10.1016/0165-3806(84)90057-9 - 21.
Gower-Winter SD, Corniola RS, Morgan TJJ, Levenson CW. Zinc deficiency regulates hippocampal gene expression and impairs neuronal differentiation. Nutritional Neuroscience. 2013; 16 :174-182. DOI: 10.1179/1476830512Y.0000000043 - 22.
Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. International Review of Neurobiology. 1989; 31 :145-238. DOI: 10.1016/s0074-7742(08)60279-2 - 23.
Blakemore LJ, Trombley PQ. Zinc as a neuromodulator in the central nervous system with a focus on the olfactory bulb. Frontiers in Cellular Neuroscience. 2017; 11 :297. DOI: 10.3389/fncel.2017.00297 - 24.
Qian J, Noebels JL. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3-CA1 synapse. The Journal of Neuroscience. 2006; 26 :6089-6095. DOI: 10.1523/JNEUROSCI.0475-06.2006 - 25.
Li Y, Hough CJ, Suh SW, et al. Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. Journal of Neurophysiology. 2001; 86 :2597-2604. DOI: 10.1152/jn.2001.86.5.2597 - 26.
Ketterman JK, Li YV. Presynaptic evidence for zinc release at the mossy fiber synapse of rat hippocampus. Journal of Neuroscience Research. 2008; 86 :422-434. DOI: 10.1002/jnr.21488 - 27.
Bitanihirwe BKY, Cunningham MG. Zinc: The brain’s dark horse. Synapse. 2009; 63 :1029-1049. DOI: 10.1002/syn.20683 - 28.
Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Research Reviews. 2009; 59 :347-373. DOI: 10.1016/j.brainresrev.2008.10.003 - 29.
Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994; 42 :393-441. DOI: 10.1016/0301-0082(94)90082-5 - 30.
Mayer ML, Vyklicky LJ. The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. The Journal of Physiology. 1989; 415 :351-365. DOI: 10.1113/jphysiol.1989.sp017725 - 31.
Frederickson CJ, Koh J-Y, Bush AI. The neurobiology of zinc in health and disease. Nature Reviews. Neuroscience. 2005; 6 :449-462. DOI: 10.1038/nrn1671 - 32.
Vogler NW, Betti VM, Goldberg JM, Tzounopoulos T. Mechanisms underlying long-term synaptic zinc plasticity at mouse dorsal Cochlear nucleus glutamatergic synapses. The Journal of Neuroscience. 2020; 40 :4981-4996. DOI: 10.1523/JNEUROSCI.0175-20.2020 - 33.
Silvestro S, Bramanti P, Mazzon E. Role of quercetin in depressive-like behaviors: Findings from animal models. Applied Science. 2021; 11 :7116 - 34.
Doboszewska U, Wlaź P, Nowak G, et al. Zinc in the monoaminergic theory of depression: Its relationship to neural plasticity. Neural Plasticity. 2017; 2017 :3682752. DOI: 10.1155/2017/3682752 - 35.
Maserejian NN, Hall SA, McKinlay JB. Low dietary or supplemental zinc is associated with depression symptoms among women, but not men, in a population-based epidemiological survey. Journal of Affective Disorders. 2012; 136 :781-788. DOI: 10.1016/j.jad.2011.09.039 - 36.
Milton AH, Vashum KP, McEvoy M, et al. Prospective study of dietary zinc intake and risk of cardiovascular disease in women. Nutrients. 2018; 10 :38. DOI: 10.3390/nu10010038 - 37.
Młyniec K, Budziszewska B, Holst B, et al. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. The International Journal of Neuropsychopharmacology. 2014; 18 :3. DOI: 10.1093/ijnp/pyu002 - 38.
Starowicz G, Jarosz M, Frąckiewicz E, et al. Long-lasting antidepressant-like activity of the GPR39 zinc receptor agonist TC-G 1008. Journal of Affective Disorders. 2019; 245 :325-334. DOI: 10.1016/j.jad.2018.11.003 - 39.
Petrilli MA, Kranz TM, Kleinhaus K, et al. The emerging role for zinc in depression and psychosis. Frontiers in Pharmacology. 2017; 8 :414. DOI: 10.3389/fphar.2017.00414 - 40.
Nowak G. Does interaction between zinc and glutamate system play a significant role in the mechanism of antidepressant action? Acta Poloniae Pharmaceutica. 2001; 58 :73-75 - 41.
Nowak G, Siwek M, Dudek D, et al. Effect of zinc supplementation on antidepressant therapy in unipolar depression: A preliminary placebo-controlled study. Polish Journal of Pharmacology. 2003; 55 :1143-1147 - 42.
Howland JG, Wang YT. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Progress in Brain Research. 2008; 169 :145-158. DOI: 10.1016/S0079-6123(07)00008-8 - 43.
Frederickson CJ, Suh SW, Silva D, et al. Importance of zinc in the central nervous system: The zinc-containing neuron. The Journal of Nutrition. 2000; 130 :1471S-1483S. DOI: 10.1093/jn/130.5.1471S - 44.
Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic dysfunction in schizophrenia: From basic neuroscience to clinical psychopharmacology. European Neuropsychopharmacology. 2008; 18 :773-786. DOI: 10.1016/j.euroneuro.2008.06.005 - 45.
Szewczyk B, Poleszak E, Sowa-Kućma M, et al. The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids. 2010; 39 :205-217. DOI: 10.1007/s00726-009-0412-y - 46.
Nowak G. Zinc, future mono/adjunctive therapy for depression: Mechanisms of antidepressant action. Pharmacological Reports. 2015; 67 :659-662. DOI: 10.1016/j.pharep.2015.01.015 - 47.
Szewczyk B, Pochwat B, Rafało A, et al. Activation of mTOR dependent signaling pathway is a necessary mechanism of antidepressant-like activity of zinc. Neuropharmacology. 2015; 99 :517-526. DOI: 10.1016/j.neuropharm.2015.08.026 - 48.
Swardfager W, Herrmann N, Mazereeuw G, et al. Zinc in depression: A meta-analysis. Biological Psychiatry. 2013; 74 :872-878. DOI: 10.1016/j.biopsych.2013.05.008 - 49.
Siwek M, Dudek D, Paul IA, et al. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. Journal of Affective Disorders. 2009; 118 :187-195. DOI: 10.1016/j.jad.2009.02.014 - 50.
Prasad AS. Zinc: Role in immunity, oxidative stress and chronic inflammation. Current Opinion in Clinical Nutrition and Metabolic Care. 2009; 12 :646-652. DOI: 10.1097/MCO.0b013e3283312956 - 51.
Lenz KM, Nelson LH. Microglia and beyond: Innate immune cells As regulators of brain development and behavioral function. Frontiers in Immunology. 2018; 9 :698. DOI: 10.3389/fimmu.2018.00698 - 52.
Kauppinen TM, Higashi Y, Suh SW, et al. Zinc triggers microglial activation. The Journal of Neuroscience. 2008; 28 :5827-5835. DOI: 10.1523/JNEUROSCI.1236-08.2008 - 53.
Bakker E, Bühlmann P, Pretsch E. Carrier-based ion-selective electrodes and bulk Optodes. 1. General Characteristics. Chemical Reviews. 1997; 97 :3083-3132. DOI: 10.1021/cr940394a - 54.
Bühlmann P, Pretsch E, Bakker E. Carrier-based ion-selective electrodes and bulk Optodes. 2. Ionophores for potentiometric and optical sensors. Chemical Reviews. 1998; 98 :1593-1688. DOI: 10.1021/cr970113+ - 55.
Ding W-Q, Lind SE. Metal ionophores—An emerging class of anticancer drugs. IUBMB Life. 2009; 61 :1013-1018. DOI: 10.1002/iub.253 - 56.
Harbison-Price N, Ferguson SA, Heikal A, Taiaroa G, Hards K, Nakatani Y, et al. Multiple bactericidal mechanisms of the zinc ionophore PBT2. mSphere. 2022; 5 :e00157-e00120. DOI: 10.1128/mSphere.00157-20 - 57.
Samutprasert P, Chiablaem K, Teeraseranee C, et al. Epigallocatechin gallate-zinc oxide co-crystalline nanoparticles as an anticancer drug that is non-toxic to normal cells. RSC Advances. 2018; 8 :7369-7376. DOI: 10.1039/C7RA10997K - 58.
Steinrauf LK, Hamilton JA, Sabesan MN. Crystal structure of valinomycin-sodium picrate. Anion effects on valinomycin-cation complexes. Journal of the American Chemical Society. 1982; 104 :4085-4091. DOI: 10.1021/ja00379a008 - 59.
Abbott BJ, Fukuda DS, Dorman DE, et al. Microbial transformation of A23187, a divalent cation ionophore antibiotic. Antimicrobial Agents and Chemotherapy. 1979; 16 :808-812. DOI: 10.1128/AAC.16.6.808 - 60.
Raatschen N, Wenzel M, Ole Leichert LI, et al. Extracting iron and manganese from bacteria with ionophores—A mechanism against competitors characterized by increased potency in environments low in micronutrients. Proteomics. 2013; 13 :1358-1370. DOI: 10.1002/pmic.201200556 - 61.
Xue J, Moyer A, Peng B, et al. Chloroquine is a zinc ionophore. PLoS One. 2014; 9 :e109180. DOI: 10.1371/journal.pone.0109180 - 62.
Aggett PJ, Delves HT, Harries JT, Bangham AD. The possible role of diodoquin as a zinc ionophore in the treatment of acrodermatitis enteropathica. Biochemical and Biophysical Research Communications. 1979; 87 :513-517. DOI: 10.1016/0006-291x(79)91825-4 - 63.
Lanke K, Krenn BM, Melchers WJG, et al. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. The Journal of General Virology. 2007; 88 :1206-1217. DOI: 10.1099/vir.0.82634-0 - 64.
Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, et al. Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1-6 cells to a liposome model. Journal of Agricultural and Food Chemistry. 2014; 62 :8085-8093. DOI: 10.1021/jf5014633 - 65.
Krenn BM, Gaudernak E, Holzer B, et al. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. Journal of Virology. 2009; 83 :58-64. DOI: 10.1128/JVI.01543-08 - 66.
Sun Q, Jia N, Ren F, Li X. Grape seed proanthocyanidins improves depression-like behavior by alleviating oxidative stress and NLRP3 activation in the hippocampus of prenatally-stressed female offspring rats. Journal of Histotechnology. 2021; 44 :90-98. DOI: 10.1080/01478885.2020.1861907 - 67.
Bohlmann L, De Oliveira DMP, El-Deeb IM, et al. Chemical synergy between Ionophore PBT2 and zinc reverses antibiotic resistance. MBio. 2018; 9 :e02391-e02318. DOI: 10.1128/mBio.02391-18 - 68.
te Velthuis AJW, van den Worm SHE, Sims AC, et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens. 2010; 6 :e1001176. DOI: 10.1371/journal.ppat.1001176 - 69.
Mizui T, Yamashina S, Tanida I, et al. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. Journal of Gastroenterology. 2010; 45 :195-203. DOI: 10.1007/s00535-009-0132-9 - 70.
Qiu M, Chen Y, Chu Y, et al. Zinc ionophores pyrithione inhibits herpes simplex virus replication through interfering with proteasome function and NF-κB activation. Antiviral Research. 2013; 100 :44-53. DOI: 10.1016/j.antiviral.2013.07.001 - 71.
de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial Agents and Chemotherapy. 2014; 58 :4875-4884. DOI: 10.1128/AAC.03011-14 - 72.
Tsai WP, Nara PL, Kung HF, Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Research and Human Retroviruses. 1990; 6 :481-489. DOI: 10.1089/aid.1990.6.481 - 73.
Romanelli F, Smith KM, Hoven AD. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Current Pharmaceutical Design. 2004; 10 :2643-2648. DOI: 10.2174/1381612043383791 - 74.
Li C, Zhu X, Ji X, et al. Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. eBioMedicine. 2017; 24 :189-194. DOI: 10.1016/j.ebiom.2017.09.034 - 75.
Yoshino S, Hara A, Sakakibara H, et al. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-a reaction in mouse brain mitochondria. Nutrition. 2011; 27 :847-852. DOI: 10.1016/j.nut.2010.09.002 - 76.
Sakakibara H, Yoshino S, Kawai Y, Terao J. Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Bioscience, Biotechnology, and Biochemistry. 2008; 72 :94-100. DOI: 10.1271/bbb.70454 - 77.
Rojas P, Serrano-García N, Medina-Campos ON, et al. Antidepressant-like effect of a Ginkgo biloba extract (EGb761) in the mouse forced swimming test: Role of oxidative stress. Neurochemistry International. 2011; 59 :628-636. DOI: 10.1016/j.neuint.2011.05.007 - 78.
Bondi CO, Rodriguez G, Gould GG, et al. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008; 33 :320-331. DOI: 10.1038/sj.npp.1301410 - 79.
Gold PW. The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry. 2015; 20 :32-47. DOI: 10.1038/mp.2014.163 - 80.
Wang J, Xu S, Chen X, et al. Antidepressant effect of EGCG through the inhibition of hippocampal neuroinflammation in chronic unpredictable mild stress-induced depression rat model. Journal of Functional Foods. 2020; 73 :104106. DOI: 10.1016/j.jff.2020.104106 - 81.
Hoang BX, Han B. A possible application of hinokitiol as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and viral infections. Medical Hypotheses. 2020; 145 :110333. DOI: 10.1016/j.mehy.2020.110333 - 82.
Troyer EA et al. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020; 87 :34-39. DOI: 10.1016/j.bbi.2020.04.027 - 83.
Kang L, Ma S, Chen M, et al. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: A cross-sectional study. Brain, Behavior, and Immunity. 2020; 87 :11-17. DOI: 10.1016/j.bbi.2020.03.028 - 84.
Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Network Open. 2020; 3 (3):e203976. DOI: 10.1001/jamanetworkopen.2020.3976 - 85.
Liu D, Baumeister RF, Zhou Y. Mental health outcomes of coronavirus infection survivors: A rapid meta-analysis. Journal of Psychiatric Research. 2021; 137 :542-553. DOI: 10.1016/j.jpsychires.2020.10.015 - 86.
Renaud-Charest O, Lui L, Eskander S, Ceban F, Ho R, Di Vincenzo JD, et al. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. Journal of Psychiatric Research. 2021; 144 :129-137. DOI: 10.1016/j.jpsychires.2021.09.054 - 87.
Jothimani D et al. COVID-19: Poor outcomes in patients with zinc deficiency. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2020; 100 :343-349. DOI: 10.1016/j.ijid.2020.09.014 - 88.
Ali N et al. Assessment of the role of zinc in the prevention of COVID-19 infections and mortality: A retrospective study in the Asian and European population. Journal of Medical Virology. 2021; 93 (7):4326-4333. DOI: 10.1002/jmv.26932 - 89.
Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. The American Journal of Emergency Medicine. 2020; 38 (9):1722-1726. DOI: 10.1016/j.ajem.2020.05.073 - 90.
Amani R, Saeidi S, Nazari Z, Nematpour S. Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biological Trace Element Research. 2010; 137 :150-158 - 91.
World Health Organization. The world health report 2002. Midwifery. 2003; 19 :72-73. DOI: 10.1054/midw.2002.0343 - 92.
Cereda G, Ciappolino V, Boscutti A, et al. Zinc as a neuroprotective nutrient for COVID-19-related neuropsychiatric manifestations: A literature review. Advances in Nutrition. 2022; 13 (1):66-79. DOI: 10.1093/advances/nmab110 - 93.
te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and Arterivirus RNA polymerase activity In vitro and zinc Ionophores block the replication of these viruses in cell culture. PLoS Pathogens. 2010; 6 (11):e1001176. DOI: 10.1371/journal.ppat.1001176 - 94.
Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. Journal of Medical Microbiology. 2020; 69 (10):1228-1234. DOI: 10.1099/jmm.0.001250