Permissible limit of heavy metal ions in water [21].
Abstract
Water has become a major threat in today’s world. Collection of heavy metals, a few of them, is potentially toxic and these get distributed to different areas through different pathways. With an increase in the earth’s population, development and industrialization are taking place rapidly and these get the major source of water contamination. With heavy metals in lakes, rivers, groundwater, and various water sources, water gets polluted by the increased concentration of heavy metals and metalloids through release from the suddenly mine tailings, disposal of high metal wastes, growing industrial areas, leaded gasoline and paints, usage of fertilizers inland, animal manures, E-waste, sewage sludge, pesticides, wastewater irrigation, coal, etc. Exposure to heavy metals has been linked to chronic and acute toxicity, which develops retardation; neurotoxicity can damage the kidneys, lead to the development of different cancers, damage the liver and lungs; bones can become fragile; and there are even chances of death in case of huge amount of exposure. This chapter mainly focuses on heavy metal pollution in water and its toxic effect on living organisms.
Keywords
- heavy metal
- water
- toxicity
- pollution
- living organisms
1. Introduction
Expeditious expansion and industrial development near the rivers have led to more stress on the river, and with increased stress, the water becomes polluted, and worsening environmental health is observed [1]. The water-soil interface and the water-atmosphere interface are the medium through which the heavy metals travel [2, 3]. Both anthropogenic activities and geochemical processes are responsible for heavy metal contamination in ecosystems [4]. Elements that have high density and are less noxious are known as heavy metals. Examples of heavy metals are lead, iron, mercury, cadmium, zinc, arsenic, copper, and chromium and the actual volume of these heavy metals is more than 6 g/m3 [5]. Heavy metals have the property of environmental persistence and bioaccumulation, and these heavy metals enter the aquatic system through various routes. These heavy metals not only impair the quality of the aquatic ecosystem but also human health [6, 7]. These heavy metals can be found on the layer of earth in their regular form. These heavy metals are so dangerous that they cannot be degraded or decomposed and they have the arability to bioaccumulate [8]. These heavy metals once get into the ecosystem through the air, via drinkable water, or multiple chemicals and products that are manmade. The route of administration of these heavy metals is via inhalation, ingestion, and skin absorption. These heavy metals get into the biosphere via human activities, which include industrial production, mining, agriculture, and transportation [9]. Some methods are fossil fuel burning, smelting of different, waste from the municipality, fertilizers, pesticides, and sewage these all are considered to be the primary sources of metal pollution [10, 11, 12, 13]. The toxicity of these heavy metals in the human body reduces energy levels; disrupts brain functioning; disturbs the functioning of various other organs such as the brain, lungs, liver, and kidney; and also hinders blood composition. If the contact with heavy metals continues, then it can hinder the physical, neurological, and muscular functioning. And due to these diseases like multiple sclerosis, Parkinson’s disease and muscular dystrophy, and Alzheimer’s disease. Chronic exposure to some of the heavy metals and their compounds may even cause cancer [14]. Pollution of these heavy metals into the river may cause distressing effects on the ecological balance of the aquatic environment, and with the extent of contamination, the diversity of aquatic organisms becomes limited [15]. The fish in the aquatic ecosystem can be used for examining the well-being of biota. Due to pollutants in the food chain of organisms, harmful effects can be seen and the aquaculture can become dead [16]. These heavy metals are neurotoxins for the fish living in the aquatic environment. When these heavy metals enter the fish body, they interact with them to generate biochemical reaction inside the fish, which makes it difficult for fish to communicate with their surroundings [17]. The presence of these heavy metals leads to diseases like Minamata, which is organic mercury poisoning. When these heavy metals get bioaccumulated, they become a threat to both the human population and animals who uses that water [18]. Modeling of risk assessment is divided into four stages, i.e., exposure assessment, toxicity (dose-response) assessment, hazard identification, and risk characterization. There are three pathways through which humans get exposed to traced metals, which include directly ingesting, inhaling through the mouth or nose, and via skin absorption when it gets exposed. From the water, the heavy metals usually enter through ingestion and dermal absorption. To assess exposure, the average daily dose is measured for pollutants through different identified paths. In a dose-response assessment for no carcinogens, reference doses (RfD) are calculated, and for carcinogens, slope factors (SF) are obtained by the United States Environment Protection Agency (USEPA) Integrated Risk Information System (IRIS) database. With the help of the facts which are discussed above, there was a study done with an aim to evaluate the water quality of the Subarnarekha River relating to metals, their temporal classification, source of identification, and assessment of human health risk when that water was ingested or the contaminate when absorbed through the skin. Through this, it is possible to know the contamination level and accordingly, the strategies were planned (Table 1) [19, 20].
| Heavy metal ions | WHO’s permissible limit (mg L−1) |
|---|---|
| Se | 0.02 |
| Hg | 0.001 |
| Mn | 0.02 |
| Ag | 0.1 |
| Cd | 0.05 |
| Cr | 0.003 |
| Pb | 0.01 |
| Zn | 3.00 |
| Fe | 0.30 |
| Cu | 0.02 |
| As | 0.01 |
Table 1.
2. Source of contamination in water
The presence of these heavy metals on the surface of the water can be due to natural or anthropogenic activities. In natural activities, weathering of rocks that contain metals, an eruption from volcanos, fires in the forest, and naturally occurring processes of weathering can be included. From these activities, metal enters the different sections of the environment. Heavy metals can be found in the forms of sulfates, hydroxides, oxides, sulfides, phosphates, and silicates [12, 22]. A huge amount of accumulation of heavy metals into the water is mainly due to anthropogenic and natural activities. Some more examples of natural source through which heavy metals contaminates water are, wet and dry deposition of atmospheric salts, water-rock interaction, or water interaction with the soil. While the sudden increase in urbanization and industrialization are an example of anthropogenic sources through which water get contaminated (Table 2; Figure 1) [23].
| Heavy metal ion | Common sources |
|---|---|
| Copper (Cu) | Fertilizers, tanning, and photovoltaic cells |
| Zinc (Zn) | Soldering, cosmetics, and pigments |
| Silver (Ag) | Refining of copper, gold, nickel, zinc, jewelry, and electroplating industries |
| Chromium (Cr) | Leather industry, tanning, and chrome plating industries |
| Arsenic (As) | Wooden electricity poles that are treated with arsenic-based preservatives, pesticides, fertilizers, the release of untreated effluents, oxidation of pyrite (FeS) and arsenopyrite (FeAsS) |
| Mercury (Hg) | Combustion of coal, municipal solid waste incineration, and volcanic emissions |
| Cadmium (Cd) | Paints, pigments, electroplated parts, batteries, plastics, synthetic rubber, photographic and engraving process, photoconductors, and photovoltaic cells |
| Lead (Pb) | PVC pipes in sanitation, agriculture, recycled PVC lead paints, jewelry, lead batteries, lunch boxes, etc. |
Table 2.
Major sources of some heavy metal ions in water [24].
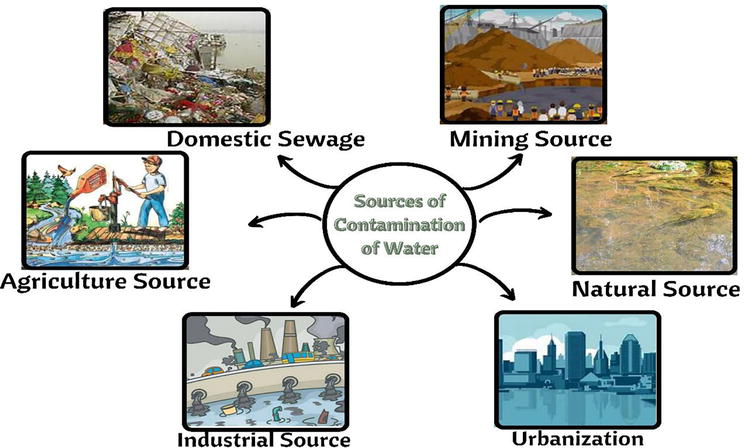
Figure 1.
Contamination of water through different sources.
2.1 Natural sources
Trace metals are found in excess levels in the environment, they are formed by geographical processes such as volcanic eruptions, weathering of rocks, and leaching into rivers, lakes, and oceans due to the action of water [25]. The presence of heavy metals in water depends on the local geology, hydrogeology, and geochemical characteristics of the aquifer [26]. One of the main sources of pollution is weathering. The weathering of the sedimentary rocks such as limestone or dolomite or shale makes the water contaminated or polluted. When there is an interaction of water with rock element, it also leads addition of these elements into the water; thus, contamination occurs. Examples of such elements are granite, syenite, basalt, gabbro, nepheline, and andesite. Due to the particular ore or the minerals, the element level increases. Elements examples are magnetite, hematite, goethite, siderite, calcite, cuprite, malachite, azurite, chromite, kaolinite, montmorillonite, arsenic trioxide, orpiment, arsenopyrite, calamine, smithsonite, pyrolusite, and rhodochrosite [27, 28, 29, 30]. The sulfide deposition also increases as it is associated with the mineralization of the gold and hydrous iron oxide ores [31].
2.2 Anthropogenic sources
Anthropogenic events, in which human settlement replaced the natural forest and agricultural activities have increased the environmental impacts. Such activities have contaminated the aquatic ecosystems, which include spring waters from the river like the Amala and Nyangores, tributaries of Mara River, Indonesia in Mau Complex. The maximum of forest land is converted into human settlement and agriculture. People who live near the Mara River Basin use that spring water for the purpose of animal and agricultural purposes [21]. The water carrying capacity has decreased with the rapid increase in industrialization and urbanization. Hg concentration in water has increased with agriculture activities and human activities. Activities like domestic sewage into the water, solid waste burning, coal and oil combustions, and pyrometallurgical processes and mining are the main reason for this. Water, by either snow or rain, brings the contaminated soil with Hg into the adjacent water areas [32, 33]. The source of Ni is the corroded metal pipes and containers [34]. The major source of lead in water majorly comes from additives of paints and petrol and aerosol precipitation, which is formed due to the high temperature used in industrial processes for the purpose of coal combustion, smelting, and cement production [35], and chloralkali, batteries, fluorescent lamps, thermometers, and electronic switches production. Chemical industries are some industrial activities through which Hg pollutes the water system and these activities are the largest contributor to Hg contamination in the environment [36].
2.3 Domestic sewage
Huge amount of untreated sewage from domestic is thrown into the river. This untreated waste from domestic has the presence of toxins. These toxins are due to the presence of solid waste or from the litter of plastic, or the contamination of bacteria due to the presence of these the water can get polluted. Domestic untreated water is thrown directly into the water resource and this majorly causes pollution inside the water and harms the ecosystem [37]. These pollutants majorly depend upon what kind of industry has thrown those pollutants. When these toxic metals get inside the water, they decrease the quality of the water [38]. Around 25% of pollution inside the water is caused only by these industries [39]. When the water gets contaminated, the water gets enriched by the nitrogen and phosphorous elements. With the presence of these nutrients, the growth rate of algae gets multiplied, and then it competed with the surrounding aquatic biota for the dissolved oxygen in water [40]. The presence of nitrite and nitrate anions leads to a major threat to the exposed organisms; examples of such threats are methemoglobinemia. It is more common in small children, and the symptoms caused by this are cyanotic color in the skin due to blood alterations [41]. Water sources that get deposited by this sewage also become anions rich, due to the presence of chlorine in urine, and NaCl is used in the human diet. On the side of the sea, there is the presence of chloride in high concentrations due to the leakage of salt into the sewerage system. It also may be increased by industrial processes [42].
2.4 Industrial source
Contamination of heavy metals in the aquatic environment is very harmful since these elements cannot be degraded and they get accumulated inside the living organisms [43]. Residue from the industry is the major source through which these heavy metals get into the aquatic ecosystems, and their accumulation in water varies with the type of wastewater treatment used [44]. Effects known as deleterious can be observed when the metal particles are introduced into the water system [45, 46]. Different metals from the Amazon River (Brazil) and the Yukon River (Alaska) were analyzed in the solid-state only. Plants have the presence of these metals in water. In tissue, the concentration of several metals is slow, and their concentration should be kept in less range only as more concentration can be harmful to the biological development of the pant [47]. Through the food chain, fish contaminants can reach man [48]. Effluent from industries, water tank leakages, dumping beside marines, and due to radioactive waste and atmospheric deposition, are some sources of water contamination. Disposed of heavy metals and waste from industries they get accumulated in rivers and lakes thus causing harmful impacts on animals and humans. For suppression of the immune, reproductive failure and acute poisoning toxins are responsible [49]. Then there is direct damage to plant or animal nutrition at that time human health is affected. The pollutants that are polluting the water are killing marine organisms such as mollusks, marine birds, fishes, and other organisms that live in the sea [50].
2.5 Urbanization
With an increase in the population has created many issues and one of the issues is the pollution of water [38]. An increase in the population leads automatically leads to more generations of solid waste [51]. Both solid waste and liquid waste are deposited into the water without any treatment. Human excreta also contaminate the water. Thus, contaminated water leads to a generation of a large number of bacteria, which is a threat to human well-being [39]. Government is unable to supply vital requirements to the People because of the increase in the number of people. Facility for sanitization is more in urban areas as compared to rural areas. Plastic bag and waste are a major contribution to pollution. People throw the waste in plastic bags into water sources [24]. From the research, it was found that around three crore people of the population defecate in the open, while 77% population use flush and around 8% use the pit latrines. Urbanization can cause many infectious diseases. Overpopulation, unhealthy conditions, and dangerous drinkable water are these major health problems in urban areas. One-third of urban people are vulnerable to disease [37].
2.6 Agriculture source
The population in rural areas is less but the use of fertilizers, pesticides, and eroded soil contaminates the water. When it rains the water from the surface runoff and that rainwater enters the nearby water resource and thus pollutes the existing water [52]. Agricultural runoff cases freshwater bodies’ eutrophication. Half of the lakes in the US are eutrophic. Phosphate has one of the major contributions to eutrophication. And the high concentration of phosphates promotes cyanobacteria and algae growth, which leads to the excessive use of the biologically dissolved oxygen inside the water [53]. Fertilizers that are too enriched with nitrogen decrease the dissolved oxygen in rivers and coastal zones thus bringing hazardous effects to the biota. Since 2006, the nitrogen in fertilizers is being controlled in America and Northwest Europe [54]. Like pesticides, which are used as pest control, these pesticides leach into groundwater, thus polluting groundwater. The pesticides that are water-soluble leach more and the sandy soil favors the process of leaching [55].
2.7 Atmospheric source
Small pollutants particles which are present in the air, get into the water stream through the rain, when it rains these particles come down and then with the flow of water enters into the sea, thus polluting the water there. These pollutants that are present in the air usually get from the burning of fossil fuels e.g. is CO2, which combines with water and produces sulfuric acid. Sulfur dioxide, which is formed via volcanic eruption and from industries, also gets attached to a water molecule to form the sulfuric acid. When coal is combusted then also sulfuric dioxide is produced and it is also produced from petroleum products. Just like this nitrogen dioxide also combines with the water and forms the nitric acid. And with the help of rainwater, they enter the water resources (Figure 2) [52, 56, 57, 58, 59].
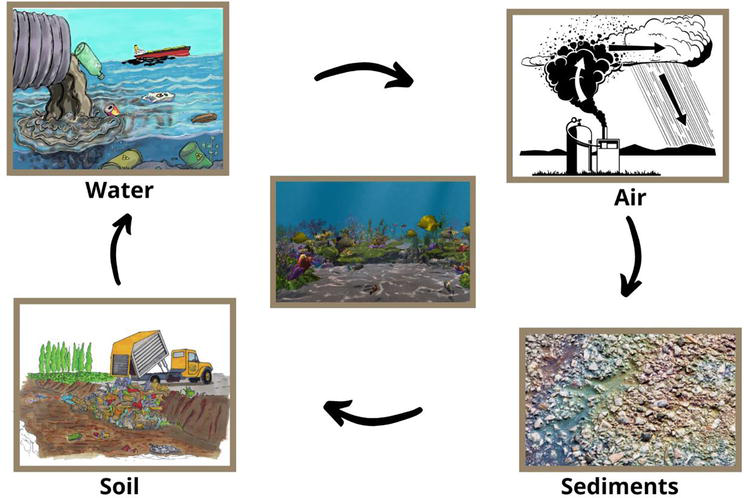
Figure 2.
Circulation of contaminants between environmental sources under the effect of atmospheric sources.
2.8 Mining source
Heavy metals are present on the earth and thus they can enter the water system through various pathways and one of them is through mining sources. When it rains or through flowing water, it leaches heavy metals out from their geological formation. These processes get disturbed when manmade economic activities such as mining are done. Through these processes, the area that is already mined out gets exposed to water and air and this leads to the acid mine drainage (AMD). The low pH conditions associated with AMD mobilize heavy metals, including radionuclides where these are present [60].
2.9 Heavy metal intake through water
Soil gets polluted with the presence of heavy metal on surface and underground water and the pollution rises when mined ores are discarded on the ground surface for manual dressing [61]. Due to the dumping over the surface, the metals get exposed to air and rain thereby generating huge AMD. If soil is polluted at that time, it gets into the plant tissue and gets accumulated there. And when those plants are grazed by animals and water is used for the drink from polluted waters, through there these heavy metals enter the body. Also, marine lives, which reproduce in contaminated water, also have the presence of heavy metals inside their body tissues, if they are lactating then inside their milk. As an overview, all organisms within a given ecosystem are contaminated via these pollutants through their food chains [62]. When nutrition from these contaminated vegetables is taken, the presence of heavy metals in those vegetables can lead to different chronic diseases. Toxic effects due to these heavy metals usually depend on the amount of concentration and the oxidative state of the particular heavy metals [63]. Heavy metals have a very dangerous impact as they are non-biodegradable in nature, have long biological half-lives, and have the potential to accumulate inside the body. Also, there are some heavy metals that are extremely toxic only because of their solubility. Fewer concentrations of heavy metals inside the food chain also show severe effects as there is no particular procedure through which these heavy metals pollutants can be extracted from the body of an organism. Nowadays presence of these toxic heavy metals is everywhere because of their extreme use in industries. In case of the wastewater, it contains a huge concentration of heavy metals, which create various health-related problems [64, 65].
3. Effect on living organism
3.1 Effect on aquatic environment
Water from estuaries and freshwater is not polluted till now to some extent, but that water is also at threat of being polluted in the long term due to metal deposition because of human past activities [66]. The water in the river and lakes can be highly polluted depending on the volume of flow and proximity to the point sources. Due to the human civilization, the element content in water is raised. Such elements are cadmium, lead, mercury, zinc, and chromium. Unlike organic chemicals, there are some metals that cannot be converted into compounds with lesser toxicity, and one of its characteristics is the loss of biodegradability. Once the heavy metals enter the water system it gets redistributed throughout the column and gets accumulated in the sediments [67]. The sediments constitute a partial contribution to polluting the natural phenomena due to their activity and metal remobilization processes. Metal residues that are present in the contaminated surroundings have the flexibility to get bioaccumulated into the aquatic environment [68]. Growth in fish larvae and juveniles is rapid. But when these heavy metals enter they might inhibit the growth rate. The fish grows in length and bulk when given the right conditions, such as a specific temperature and an acceptable amount of food. Fish growth, on the other hand, may be impeded in water contaminated with toxicants, such as heavy metals. One of the most noticeable signs of metal toxicity in fish larvae is growth inhibition. As a result, the length and bulk of fish are indications of environmental conditions [69]. Heavy metals are introduced in liquid form and surface water constituents (carbonate, sulfate, organic substances humic, fulvic, and amino acids) cause the formation of non-soluble salts or complexes. Aquatic species are not expected to be harmed by these salts and compounds. Some of them sink and collect in the sediments at the bottom. A decrease in pH of water either due to acid rain or any other acidic incidents, due to the heavy metal’s deposition into the water column, causes aquatic biota to become poisonous. Low levels of heavy metals can also make chronic stress, through fish might not get dead but can cause them to lose weight and become smaller, reducing their capacity to compete for food and habitat [70]. Pollution poses a hazard to both freshwater and marine habitats. Heavy metal poisoning of water is a significant environmental hazard that has detrimental consequences for organisms who are exposed to it be that plant-animal or humans. Fish from freshwater are majorly exposed to various heavy metals, which are added into the water bodies through the different-different sources. Contamination of these heavy metals into aquaculture has intensified global issues because it shows a risk to fish and has harmful impacts on fish buyers [71]. There are three different modes through which heavy metals enter the fish. These methods are either through the gills of fish, by the body of the fish, or by the digestive tract of the fish. Heavy metals immediately enter the fish body through the gills, while the body surface takes time for uptaking of these heavy metals through this mode [72]. Mostly the metals get accumulated in the liver, kidney, and gills. In fishes, the muscles have most of the metals present there as compared to the other body parts of the fish. Too much accumulation of these heavy metals inside the fish organ can cause lesions and operative disturbances [73]. These heavy metals also interfered with the embryo’s shape and the metabolic processes of the fishes. Structural and functional defects throughout the development of the embryo resulted in fewer larvae hatching. Several freshly born larvae die shortly after hatching owing to lead and copper absorption [74, 75]. Heavy metals get into the fish through three routes: the first is via the fish gills, the second is through the digestive tract of the fish and the last one is through the body of the fish. The gills of fish are the area that is known for the primary metal intake from the contaminated water. On the fish gills, zinc accumulates. It suggests a depressing influence on tissue respiration, which leads to hypoxia and mortality. Zinc pollution also causes alterations in the structure of the lungs and heart [76]. Humans and fish are both affected by mercury. Brain damage, with postnatal and fetal problems, leads to abortions, congenital deformity, and development differences in young fry due to Monomethyl. Minamata illness and Hg poisoning (via methyl Hg) both showed considerable neurotoxicity [77]. Nickel is necessary for tiny amounts for the formation of RBC, but when its concentration gets increased, at that time, it becomes harmful or poisonous. Cd has been linked to an increase in blood pressure and cardiac illness in fish. Blood vessels damage, hemorrhages, and depletion of blood cell count of a fish are induced by Hg, from previous research. Anemia, eosinophilia, lymphocytosis, bronchial, and renal injuries can affect chromium levels in the blood [18]. Malformations in fish are caused by cadmium, nickel, mercury, chromium, lead, and arsenic. The accumulation of these heavy metals in excessive amounts causes a variety of physiological effects. Fin loss, gill underdevelopment, liver dysfunction, and fin function in fingerlings were all prevalent findings in the studies [78]. The harmful effects of heavy metals have the greatest impact on the death rate, reproduction, individual development rates, and physiological capacity of fish. There have been effects on physical functioning and chemical parameters in the tissues and blood of fish living in water that is polluted via metals. It has been reported that fish exposed to metals developed immune system defects, making them more susceptible to infectious infections and increasing their chances of dying (Figure 3) [79].
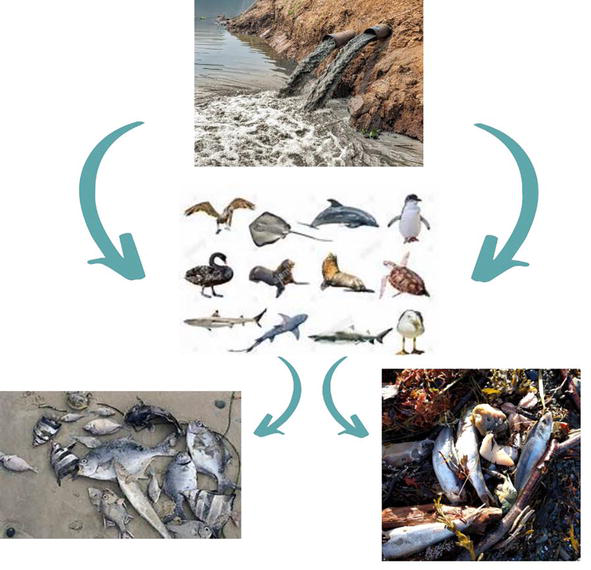
Figure 3.
Harmful effects on the aquatic environment.
3.2 Effects on aquatic plants
For the growth of plants, few HMs like As, Cd, Hg, Pb, and Se are not important as they do not perform any known physiological function in them. Others, such as Co, Cu, Fe, Mn, Mo, Ni, and Zn, are key elements that are required for regular plant development and metabolism, but their amounts can quickly exceed the appropriate levels, resulting in poisoning [80, 81]. Heavy metal concentrations in plants vary by plant species, and the efficiency with which various plants absorb metals is measured by plant absorption or metal transfer factors from soil to plant. An increased amount of Pb in agricultural soil decreases the productivity rate of the soil, and a less lead amount may hinder some important processes of plant, dark green leaves, withering of older leaves, stunted foliage, and brown short roots are poisonous indicators of photosynthesis, mitosis, and water absorption [82]. Heavy metals are poisonous and phytotoxic to plants, resulting in diseases such as chlorosis, poor plant development, and yield depression, as well as decreased nutrient absorption, plant metabolic problems, and a reduced capacity to fix molecular nitrogen in leguminous plants. Seed germination was gradually reduced in the presence of increasing levels of lead, it may be due to exposure to lead for a longer duration, some methods, such as leaching, chelation, metal binding, or microbe accumulation, have resulted in the neutralization of lead’s harmful effects [83]. Heavy metals such as Cd, Pb, and Ni even their small concentration in plants can be hazardous to them. Poisoning due to this heavy metal will result in the complex interplay between the primary unpleasant ions and additional necessary or non-essential ions. Metals affect the activity of enzymes by swapping metal ions from metal enzymes, as well as preventing plant growth [84]. Some exceptional metals are vital for plants, as they reveal their roles in the catabolism of plants and biosynthesis, together as cofactors for enzymes and as metabolic yields. For example, Zn, Fe, Cu, Cr, and Co are the important nutrients but when their amounts are increased, they become toxic. Comparatively, Pb and Cd have no effect, which is favorable to the plant and is solely lethal [85]. The most abundant hazardous elements in the soil are lead. Pb poisoning in the soil is caused by municipal sewage sludge discharge, mining and smelting operations, Pb-containing paints, paper and pulp, gasoline, and explosives. They do not have any role in the shape of the plant or their growth and photosynthetic process of the plant. Pb poisoning also inhibits enzyme action, creates an imbalance of the water, alters membrane permeability, and disrupts mineral feeding [86].
3.3 Effects on fish
One of the main sources of contamination of the water is heavy metals, as it overwhelms the important species indirectly through biological chains or directly via chemical modifications in water. Three potential ways are there, through which heavy metals get into the fish body: though fish gills, through the body of the fish, and through the fish digestive tract. Gills are responsible for the immediate absorption of metals from the water, whereas the body surface is thought to have a smaller role in the intake of these elements in fish [87]. By altering the normal activities of numerous enzymes and metabolites, the accumulation of these heavy metals in the tissues causes significant biochemical, physiological, and histological changes in fish and other freshwater fauna. Fish are one of the most widely dispersed creatures in the aquatic ecosystem, and their susceptibility to metal poisoning may indicate the extent of metal pollution’s biological impact [88]. Heavy metals, such as As, Cd, Cu, Cr, Fe, Pb, Mn, Hg, Ni, Zn, and tin (Sn), are major contaminants that cause serious toxicity in fish. Due to the heavy metals, the physiological and biochemical functions both in tissues and in blood Carpi can be altered. The compounds of As and inorganic As, Cd, Ni, silica in its crystal form, beryllium, and its compounds are considered to be chemical carcinogens, which results in the development of cancer inside the fishes. The drop in hematological parameters indicated that the exposed fishes had become anemic as a result of Cr exposure. This dangerous heavy metal was released into the aquatic ecosystem via trash, causing severe anemia and changes in hematological parameters in the
4. Conclusion
Water pollution is a global problem, and the world’s population is suffering the consequences of tainted water. Living organisms are also affected by the polluted water very much and it is very harmful to the environment. Acute and choric illnesses are caused by heavy metal concentrations in drinking water that exceed the permissible limits set by several national and international organizations. These can range from nonfatal, such as muscle and physical weakness, to fatal, such as brain, nervous system, and even cancer. Water quality testing is necessary for the protection of human health and the environment.
References
- 1.
Giri S, Singh AK. Risk assessment, statistical source identification and seasonal fluctuation of dissolved metals in the Subarnarekha River, India. Journal of Hazardous Materials. 2014; 265 :305-314 - 2.
Ali MM et al. Assessment of toxic metals in water and sediment of Pasur River in Bangladesh. Water Science and Technology. 2018; 77 (5):1418-1430 - 3.
Varol M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. Journal of Hazardous Materials. 2011; 195 :355-364 - 4.
Li GH, Cao ZM, Lan DZ, Xu J, Wang SS, Yin W. Spatial variations in grain size distribution and selected metal contents in the Xiamen Bay China. Environmental Geology. 2007; 52 (8):1559-1567 - 5.
Tavakoli-Hosseinabady B et al. Detoxification of heavy metals from leafy edible vegetables by agricultural waste: Apricot pit shell. Journal of Environmental & Analytical Toxicology. 2018; 8 (1):548 - 6.
Meng T et al. Determinants of urban consumer expenditure on aquatic products in Shanghai, China. Aquaculture Economics & Management. 2021:1-24 - 7.
Sharma M et al. Kinetics of sorption of lead on bed sediments of River Hindon, India. Environmental Monitoring and Assessment. 2009; 157 (1):11-21 - 8.
Baby J et al. Toxic effect of heavy metals on aquatic environment. International Journal of Biological and Chemical Science. 2010; 4 (4):1-39 - 9.
Sardar K et al. Heavy metals contamination and what are the impacts on living organisms. Greener Journal of Environmental Management and Public Safety. 2013; 2 (4):172-179 - 10.
Nriagu JOJN. A global assessment of natural sources of atmospheric trace metals. Nature. 1989; 338 (6210):47-49 - 11.
Nriagu JOJN. Global inventory of natural and anthropogenic emissions of trace metals to the atmosphere. Nature. 1979; 279 (5712):409-411 - 12.
Kabata-Pendias A, Pendias H. Trace Elements in Soil and Plants. London: CRC Press; 1984 - 13.
Rai PK. Technology, Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Critical Reviews in Environmental Science and Technology. 2009; 39 (9):697-753 - 14.
Ghosh L, Adhikari S, Ayyappan SJRJET. Assessment of toxic interactions of heavy metals and their effects on accumulation in tissues of fresh water fish. Research Journal of Environmental Toxicology. 2007; 1 :37-44 - 15.
Ay T et al. Bioconcentration of metals in the body muscle and gut of Clarias gariepinus exposed to sublethal concentrations of soap and detergent effluent. Journal of Cell and Animal Biology. 2009; 3 (8):113-118 - 16.
Sankhla MS, Kumar R. New and advanced technologies in aquaculture to support environmentally sustainable development. In: Microbial Biotechnology: Basic Research and Applications. Germany: Springer; 2020. pp. 249-263 - 17.
Baatrup EJCB, Pharmacology PPCC. Structural and functional effects of heavy metals on the nervous system, including sense organs, Fisheries. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 1991; 100 (1-2):253-257 - 18.
Sonone SS et al. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Letters in Applied NanoBioScience. 2020; 10 (2):2148-2166 - 19.
Means B. Risk-Assessment Guidance for Superfund. Volume 1. Human Health Evaluation Manual. Part A. Interim report (Final). Washington, DC (USA): Environmental Protection Agency; 1989 Office of Solid Waste - 20.
Wu B et al. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bulletin of Environmental Contamination and Toxicology. 2009; 82 (4):405-409 - 21.
Sankhla et al. Seasonal Variations of Lead and chromium concentration in the water samples from Yamuna River in Delhi, India. Iranian Journal of Toxicology. 2021; 15 (2):109-114 - 22.
Salgarello M, Visconti G, Barone-Adesi L. Interlocking circumareolar suture with undyed polyamide thread: A personal experience. Aesthetic Plastic Surgery. 2013; 37 (5):1061-1062 - 23.
Priti P, Paul B. Assessment of heavy metal pollution in water resources and their impacts: A review. Journal of Basic and Applied Engineering Research. 2016; 3 (8):671-675 - 24.
Gumpu MB et al. A review on detection of heavy metal ions in water–An electrochemical approach. Sensors and Actuators B: Chemical. 2015; 213 :515-533 - 25.
Bagul V et al. New perspective on heavy metal pollution of water. Journal of Chemical and Pharmaceutical Research. 2015; 7 (12):700-705 - 26.
Wang S, Mulligan CN. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution. Science of the Total Environment. 2006; 366 (2-3):701-721 - 27.
Wedepohl KH. The composition of the continental crust. Geochimica et cosmochimica Acta. 1995; 59 (7):1217-1232 - 28.
Ball JW, Izbicki JJAG. Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Applied Geochemistry. 2004; 19 (7):1123-1135 - 29.
Viers J et al. Evidence of Zn isotopic fractionation in a soil–plant system of a pristine tropical watershed (Nsimi, Cameroon). Chemical Geology. 2007; 239 (1-2):124-137 - 30.
Hüffmeyer N, Klasmeier J, Matthies M. Geo-referenced modeling of zinc concentrations in the Ruhr river basin (Germany) using the model GREAT-ER. Science of the Total Environment. 2009; 407 (7):2296-2305 - 31.
Nordstrom DKJS. Worldwide Occurrences of Arsenic in Ground Water. Washington D.C.: American Association for the Advancement of Science; 2002. pp. 2143-2145 - 32.
Kowalski A, Siepak M, Boszke L. Mercury Contamination of Surface and Ground Waters of Poznań, Poland. Polish Journal of Environmental Studies. 2007; 16 (1) - 33.
Wang Q et al. Sources and remediation for mercury contamination in aquatic systems—A literature review. Environmental Pollution. 2004; 131 (2):323-336 - 34.
Cempel M, Nikel GJPJS. Nickel: A review of its sources and environmental toxicology. Polish Journal of Environmental Studies. 2006; 15 (3):375-382 - 35.
Li Y et al. The distribution of dissolved lead in the coastal waters of the East China Sea. Marine Pollution Bulletin. 2014; 85 (2):700-709 - 36.
Li P et al. Mercury pollution in Asia: A review of the contaminated sites. Journal of Hazardous Materials. 2009; 168 (2-3):591-601 - 37.
Kamble SM. Water pollution and public health issues in Kolhapur city in Maharashtra. International Journal of Scientific and Research Publications. 2014; 4 (1):1-6 - 38.
Ho Y et al. Industrial discharge and their effect to the environment. Industrial Waste, Intech. 2012:1-39 - 39.
Desai N. SmtVanitaben. A study on the water pollution based on the environmental problem. Indian Journal of Research. 2014; 3 (12):95-96 - 40.
Moore JW, Moore EA. Resources in environmental chemistry. I. An annotated bibliography of the chemistry of pollution and resources. Journal of Chemical Education. 1976; 53 (3):167 - 41.
Sankhla et al. Impact of variation in climatic changes in concentration of Lead & Nickel in Yamuna River Water, Delhi, India. Materials Today: Proceedings. Elsevier; 2022 - 42.
Baird RB et al. Standard Methods for the Examination of Water and Wastewater. Vol. 23. Washington, DC: American Public Health Association; 2017 - 43.
Jardim WDF. Metais pesados: Um dano irreparável. Rev. Bras. Tecno. 1983; 14 (2):41-42 - 44.
Magossi L, P.J.S.P.E.M. Bonacella, Poluição das águas–coleção desafios. 9ª. 1992 - 45.
Tavares TM, Carvalho FMJQN. Avaliação de exposição de populações humanas a metais pesados no ambiente: Exemplos do recôncavo baiano. Química nova. 1992; 15 (2):147-154 - 46.
Gibbs RJ. Transport phases of transition metals in the Amazon and Yukon Rivers. Geological Society of America Bulletin. 1977; 88 (6):829-843 - 47.
Allaway W. Agronomic controls over the environmental cycling of trace elements. In: Advances in Agronomy. Netherlands: Elsevier; 1968. pp. 235-274 - 48.
Pfeiffer W et al. Metais pesados no pescado da Baía de Sepetiba, Estado do Rio de Janeiro. Ciência e Cultura. 1985; 37 (2):297-302 - 49.
Juneja T, Chaudhary A. Assessment of water quality and its effects on the health of residents of Jhunjhunu district, Rajasthan: A cross sectional study. Journal of Public Health and Epidemiology. 2013; 5 (4):186-191 - 50.
Khan MA, Ghouri AM. Environmental pollution: Its effects on life and its remedies. Researcher World: Journal of Arts, Science & Commerce. 2011; 2 (2):276-285 - 51.
Jabeen S et al. Health impact caused by poor water and sanitation in district Abbottabad. Journal of Ayub Medical College Abbottabad. 2011; 23 (1):47-50 - 52.
Letchinger M. Pollution and water quality, neighbourhood water quality assessment. Project Oceanography. 2000 - 53.
Werner W. Fertilizers, 6. Environmental aspects. Ullmann’s Encyclopedia of Industrial Chemistry. 2000 - 54.
Van Grinsven H et al. Management, regulation and environmental impacts of nitrogen fertilization in northwestern Europe under the Nitrates Directive; A benchmark study. Biogeosciences. 2012; 9 (12):5143-5160 - 55.
Ramdwar MN, Maharaj R, Siew NJSFP. Sustaining the environment: Farm and beyond the farm. 2 Sustainable Food Production: 209 - 56.
Larry W. World Water Day. A Billion people Worldwide Lack Safe Drinking. 2006 - 57.
Florescu D et al. The influence of pollution monitoring parameters in characterizing the surface water quality from Romania southern area. Romanian Journal of Physics. 2011; 56 (7-8):1001-1010 - 58.
Tietenberg T. Economics of pollution control, Chapter 15: Environmental and natural resource economics. 7th ed. New York, Boston: Pearson; 2006 - 59.
Moss B. Water pollution by agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008; 363 (1491):659-666 - 60.
Sankhla MS et al. Heavy metals contamination in water and their hazardous effect on human health—A review. International Journal of Current Microbiology and Applied Sciences. 2016; 5 (10):759-766 - 61.
Garbarino J. et al. Contaminants in the Mississippi River US Geological Survey Circular 1133. 1995 - 62.
Truby, P., Impact of Heavy Metals on Forest Trees from Mining Areas. 2003 - 63.
Duruibe JO, Ogwuegbu M, Egwurugwu JN. Heavy metal pollution and human biotoxic effects. International Journal of physical sciences. 2007; 2 (5):112-118 - 64.
Chen Y, Wang C, Wang Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environment International. 2005; 31 (6):778-783 - 65.
Singh KP et al. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere. 2004; 55 (2):227-255 - 66.
Forstner U, Wittmann GT. Metal Pollution in the Aquatic Environment. Germany: Springer-Verlag; 1979 - 67.
Meybeck M, Chapman DV, Helmer R. Global freshwater quality: A first assessment. Global Freshwater Quality: A First Assessment. 1990:306-306 - 68.
Joshi R, Ahmed SJCES. Status and challenges of municipal solid waste management in India: A review. Cogent Environmental Science. 2016; 2 (1):1139434 - 69.
Sarnowski P, Jezierska B. A new coefficient for evaluation of condition of fish Electronics. Journal of Ichthyology. 2007; 2 :69-76 - 70.
Jadhav J. et al, Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresource Technology. 2010; 101 (1):165-173 - 71.
Atici T et al. The accumulation of heavy metals (Cd, Pb, Hg, Cr) and their state in phytoplanktonic algae and zooplanktonic organisms in Beysehir Lake and Mogan Lake, Turkey. African Journal of Biotechnology. 2010; 9 (4):475-487 - 72.
Roméo M et al. Heavy metal distribution in different fish species from the Mauritania coast. Science of the Total Environment. 1999; 232 (3):169-175 - 73.
Jezierska B, Witeska M. The metal uptake and accumulation in fish living in polluted waters. In: Soil and Water Pollution Monitoring, Protection and Remediation. Germany: Springer; 2006.pp. 107-114 - 74.
Perry DM et al. Cytogenetic effects of methylmercury in embryos of the killifish, Fundulus heteroclitus. Archives of Environmental Contamination and Toxicology. 1988; 17 (5):569-574 - 75.
Jezierska B et al. The effects of heavy metals on embryonic development of fish (a review). Fish Physiology and Biochemistry. 2009; 35 (4):625-640 - 76.
Beijer K. Sources, transport and transformation of metals in the environment. Handbook of the Toxicology of Metals. 1986; 1 :68 - 77.
Pandey G, Madhuri S. Heavy metals causing toxicity in animals and fishes. Research Journal of Animal, Veterinary and Fishery Sciences. 2014; 2 (2):17-23 - 78.
Nwabunike MO. The effects of bioaccumulation of heavy metals on fish fin over two years. Global Journal of Fisheries and Aquaculture. 2016; 4 (1):281-289 - 79.
Amundsen P-A et al. Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Science of the Total Environment. 1997; 201 (3):211-224 - 80.
Garrido S et al. Heavy metals in soil treated with sewage sludge composting, their effect on yield and uptake of broad bean seeds (Vicia faba L.). Water, Air, and Soil Pollution. 2005; 166 (1):303-319 - 81.
Rascio N, Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Science. 2011; 180 (2):169-181 - 82.
Bhattacharyya P et al. Fractionation and bioavailability of Pb in municipal solid waste compost and Pb uptake by rice straw and grain under submerged condition in amended soil. Geosciences Journal. 2008; 12 (1):41-45 - 83.
Ashraf R, Ali TA. Effect of heavy metals on soil microbial community and mung beans seed germination. Pakistan Journal of Botany. 2007; 39 (2):629 - 84.
Agarwal SJA. Studies on the Effect of the Auto Exhaust Emission on the Mitragyna Patriflora. India: MDS University; 1999 - 85.
Radojevic M, Bashkin V.J.U.K. The Royal Society of Chemistry Cambridge. 1999 - 86.
Sharma P, Dubey RS. Lead toxicity in plants. Brazilian Journal of Plant Physiology. 2005; 17 :35-52 - 87.
Tekin Özan S, Aktan NJJZ. Relationship of heavy metals in water, sediment and tissues with total length, weight and seasons of cyprinus carpio L, 1758 From Işikli Lake (Turkey). Journal of Zoology. 2012; 44 (5):1405-1416 - 88.
Vanisree et al. Heavy metal contamination of food crops: Transportation via food chain, human consumption, toxicity and management strategies. Environmental Impact and Remediation of Heavy Metals. Intech Open; 2022 - 89.
Praveena M et al. Impact of tannery effluent, chromium on hematological parameters in a fresh water fish, Labeo Rohita (Hamilton). Research Journal of Animal, Veterinary and Fishery Sciences. 2013; 1 (6):1-5 - 90.
Allen P. Accumulation profiles of lead and cadmium in the edible tissues of Oreochromis aureus during acute exposure. Journal of Fish Biology. 1995; 47 (4):559-568 - 91.
Johnson LL et al. Patterns of oocyte development and related changes in plasma 17-β estradiol, vitellogenin, and plasma chemistry in English sole Parophrys vetulus Girard. Journal of Experimental Marine Biology and Ecology. 1991; 152 (2):161-185 - 92.
Lye C, Frid C, Gill MJMEPS. Seasonal reproductive health of flounder Platichthys flesus exposed to sewage effluent. Marine Ecology Progress Series. 1998; 170 :249-260 - 93.
Vosylienė MZ, Mikalajūnė AJE. Effect of heavy metal model mixture on rainbow trout biological parameters. Ekologija. 2006; 4 :12-17 - 94.
Landolph JR. Molecular mechanisms of transformation of C3H/10T1/2 C1 8 mouse embryo cells and diploid human fibroblasts by carcinogenic metal compounds. Environmental Health Perspectives. 1994; 102 (suppl. 3):119-125