Clinical studies involving monolayer and multilayer coatings as well as the OxZr surface modification.
Abstract
All metal implants in human bodies corrode, which results in metal ion release. This is not necessarily a problem and represents for most patients no hazard. However, both local and systemic effects are possible, including hypersensitivity. To avoid this, coatings on standard implants (mono- or multi-layer) and surface modifications have been developed and are in use. This chapter explains the background of metal ion release, biological reactions, coating technologies, biotribological and biomechanical properties, as well as the clinical results of modern knee arthroplasty implant coatings. There is no general concern about metal ion release from CoCrMo standard implants for most patients. If patients present with a confirmed metal allergy, a multilayer-coated or oxidized zirconium implant is currently the best option for these patients.
Keywords
- metal ion release
- hypersensitivity
- coating
- multilayer
- biological effects
1. Introduction
Total knee arthroplasty (TKA) is a very effective treatment option for advanced osteoarthritis of the knee with overall low revision rates [1]. However, not all patients benefit from the surgery and not all problems have been solved. One issue is the biological reaction to implanted materials. Knee arthroplasty implants are usually made of cobalt-chromium alloys. Material from implants is always released after implantation, either as a result of mechanical (wear) or electrochemical processes (corrosion). Biological reactions in patients, caused by the release of metal particles and metal ions, have been reported (allergy, inflammatory response). Furthermore, cobalt, a relevant part of the implant alloy, has recently been listed as a potentially carcinogenic and mutagenic category 1B hazard by the European Commission. To overcome these potential issues, the surface of knee arthroplasty implants has been coated or ceramised. Besides a reduction in the release of metal ions and metallo-organic complexes, this results additionally in better wear performance. Unfortunately, there have been reports about less favorable results with such hypoallergenic materials [2, 3, 4]. In a retrieval study, 21% of the TKAs demonstrated coating delamination, which might affect the performance of the coating [4].
This chapter explains the background of metal ion release, current coating technologies being used in modern knee arthroplasty implants, their biomechanical properties, biological reactions and clinical results.
2. Biological reactions
2.1 Metal ion release
All metal implants in human bodies corrode which results in metal ion release. This represents for most patients no problem. However, if a critical metal ion concentration is exceeded, local or rarely systemic problems can occur. The released metal particles may accumulate in and around affected joints as well as in body fluids, lymph nodes, bone marrow and internal organs. As a result, both local and systemic reactions are possible. Local tissue reactions to metal particles around joint arthroplasties have been described in the past by the generic term “metallosis”. Natu et al. [5] introduced the term “adverse reactions to metal debris” (ARMD) which is the most widely used description for local tissue reactions.
Potential systemic adverse effects of metal particles include toxicity-related organ damage, mutagenic effects (carcinogenesis) and teratogenicity. Discussions have primarily focused on the potential damage of cobalt and chromium. While chromium particles may be significant in terms of their mutagenic potential, cobalt has been described to cause organ toxicity. In a review, Leyssens et al. [6] described the potential damage from cobalt exposure with special reference to different routes of exposure (including metallic bearings). The highest concentrations of cobalt in the body were seen in oral uptake and failed metal implants. The clinically possible sequelae of cobalt intoxication include mainly neurological effects (particularly impaired hearing and eyesight) as well as cardiovascular and endocrine effects. In general, it is assumed that such cobalt-related effects on organs require concentrations higher than 300 μg/l, which have not been described in patients with standard knee arthroplasty implants [7, 8].
Another potential effect of metal ions may be chromosomal aberrations. Therefore, the potential carcinogenic effects of metal implants have been discussed. However, in two extensive meta-analysis, no evidence of an increase in systemic tumor incidence was found. Considering the available clinical data including large registry studies, there is no evidence that metal-containing arthroplasty implants increase the risk of cancer or mortality in patient [9]. The same applies to potential teratogenic effects, which have not been reported.
Both, local and systemic effects are mostly a problem of metal-on-metal (MoM) hip arthroplasties or dual taper modular hip stems [10, 11, 12, 13, 14] but rarely seen in knee arthroplasties. Only large knee arthroplasty implants (tumor implants, hinged TKA, MoM) have the potential to cause relevant metal ion release and therefore local or systemic effects [15]. In standard TKA implants, this is not a matter of concern. Several studies have investigated metal ion concentrations in standard and coated TKA [16]. Although a relevant reduction of metal ion release has been reported in-vitro [17], there have been no relevant differences in-vivo. However, there are reports about the relevant increase in large hinged TKA which is a matter of concern [15] and needs to be observed.
2.2 Hypersensitivity and immunological reactions
There is a debate if patients with skin allergies to implant materials may react to metal implants, resulting in incompatibility reactions including persistent swelling, pain and early loosening. Moreover, there is still a controversy if hypoallergenic implants may be advantageous for these patients [18, 19, 20, 21, 22]. Despite these controversies, the prevalence of contact allergies against implant materials is high (especially nickel), and affected patients usually ask for hypoallergenic implants [23].
It has been demonstrated that patients with metal implants have more often skin allergies against implant materials than patients without such implants [24]. It has been discussed that the long contact time to the metal implant may result in a hypersensitization. However, only very few patients present with symptoms. There are reports about the improvement of symptoms after the revision of standard TKA to coated TKA implants [25]. On the contrary, it has also been reported that there is no increased risk of failure when implanting a standard TKA in skin-sensitive patients [19, 26]. However, functional outcome after TKA is worse in patients with reported metal allergies [27], which might be explained by psychological aspects [28]. Even if there is to date no clear evidence that patients with reported metal allergies need hypoallergenic implants, this psychological advantage might be helpful for patient satisfaction.
Several proinflammatory cytokines have been reported to play a role in healing and pain after TKA [29, 30]. It has been demonstrated that an increased inflammatory reaction after TKA results in less favorable results [31] and that cytokines were lower after being coated compared to standard TKA [32]. Surface modification of TKA may therefore result in less inflammation and better results.
3. Coating technologies in total knee arthroplasty
Coating of metallic components, mostly applied by the physical vaporing deposition (PVD) process, was first conceived as a solution for patients with hypersensitivity reactions to cobalt and nickel, as it prevented the release of such ions from the substrate material. There are currently different coating technologies for TKA implants in use. Historically, single-layer coatings, also known as monolayer coatings, came first in clinical application. Monolayers are in clinical use in two versions: composed of titanium nitride (TiN) and titanium niobium nitride (TiNbN). TiN and TiNbN monolayer coatings are in clinical use for hip, knee, and ankle arthroplasty since the early 1990s. They are applied by means of PVD on typical orthopedic implant materials such as titanium alloy (Ti6Al4V) or cobalt chromium molybdenum alloy (CoCrMo).
Another option is the oxidized zirconium (OxZr), which is not considered as a coating technology but more a surface modification of a ZrNb2 base material [28] driven by thermal oxidation at 500°C [33]. The resulting zirconium oxide (ZrO2) is a ceramic surface with good wear characteristics and reduced release of metal ions. This material has been in clinical use for knee arthroplasty since the 1980s [34].
Further development is represented by a zirconium nitride multilayer coating, which is composed of seven layers applied via PVD and has been in clinical use since 2006. The ceramic surface is composed of zirconium nitride as the top layer, chromium nitride and chromium carbon nitride as transition layers and a bonding layer of chromium, which integrates with the base material of the implant.
4. Biocompatibility, biotribology and biomechanics
TKA was originally conceived as a procedure for elderly patients with low to moderate activity levels. As the survival rates increased to more than 90% after 10 years due to advances in the bearing materials [35, 36, 37], this procedure was expanded to younger and more active patients. However, register data has shown a decrease in the survival rate during the second and third decades of clinical performance [38, 39, 40], particularly in younger patients [38].
The most common causes for revision surgery in TKA are aseptic loosening including wear-induced osteolysis and periprosthetic infection. Aseptic loosening is a direct consequence of wear particles (metallic and polyethylene) released by the articulating surfaces, which are phagocytized by macrophages and giant cells that induce the liberation of proinflammatory cytokines (interleukins IL-1ß, IL-6, and the TNF-α), which, in turn, stimulate the osteoclasts and reduce the activity of the osteoblasts. As a result, an osteolytic activity at the implant-bone interface occurs, resulting in a loosening of the implant components. This inflammatory response is dependent on the amount of wear particles, as well as their type, size and shape [16, 22, 41, 42, 43, 44, 45]. For this reason, younger and more active patients that generate more wear particles during a longer period of time, are at a higher risk of revision due to aseptic loosening and wear [46].
One important requirement for the coatings is that they should be able to generate the same amount of wear or less as their uncoated versions and the coating should be able to withstand the whole clinical lifespan of the implant. In order to evaluate and compare the wear behavior of different knee implants, the standardized wear test ISO 14243 is performed. During this test, the knee implants are subjected to a serum-based test medium at 37°C to the motion and loading profiles of the level walking activity of a 75 kg person for a total of 5 million cycles.
Another important aspect regarding the wear behavior of the coated implants is the analysis of the metal ion and particle release, as a relationship between cobalt and chromium blood levels and failure of implants has been demonstrated [47, 48, 49, 50]. Retrieval and in vitro studies have shown that the CoCrMo femoral components develop scratches through their articulation with the ultra-high molecular weight polyethylene (UHMWPE) gliding surface and thus release metal ions [17, 51, 52, 53, 54, 55].
4.1 Biocompatibility
Zirconium material and Titanium-based coatings are considered as very biocompatible surfaces [56]. Several cell-culture and animal laboratory studies have shown that TiN coatings do not increase cell activity, show no difference to Ti6Al4V and have lower adhesion and proliferation over 24 hours of bacteria cultures than other uncoated metals [3]. Furthermore, in biocompatibility tests, TiN-coated test specimens made out of cobalt-chromium and titanium alloys did not cause any biotoxic damage [57].
Regarding ZrN multilayer coating Thomas et al. [48] performed patch tests on patients with and without metal ion hypersensitivity and showed no allergic reaction to the ZrN multilayer coated probes. Moreover, implanted multilayer-coated sticks in rabbits showed no adverse reactions to bone or tissues. Finally, a laboratory test with bacterial contamination showed 45% less biofilm formation on the ZrN multilayer coated surface in comparison to CoCrMo [58].
4.2 Biotribology and biomechanics of OxZr and TiN/TiNbN monolayers
One of the main goals of the oxidized zirconium is wear reduction, which has been tested in several wear simulation tests. The wear reduction compared to a standard CoCrMo femur against UHMWPE inserts ranges between 42% and 89%, depending on the wear simulation method, implant design and measurement protocol [59]. It also shows better scratch resistance than CoCrMo [60], as the nano-hardness of the zirconia layer ranges from 12 to 14 GPa, which is significantly harder compared to the 2–4 GPa from the CoCrMo standard material [56]. This has been demonstrated in three out of six OxZr retrieval studies from 1 to 5 years in-situ, which showed significantly less roughness of the femur component or less damage in the UHMWPE insert compared to CoCrMo implants. However, the other three retrieval studies showed no difference in the component analysis for femur and polyethylene insert damage [59].
A review of the literature on the effects of TiN coatings showed that TiN coatings have a positive effect on the biocompatibility and tribological properties of implant surfaces. TiN-coated implants showed also a high scratch resistance and less polyethylene wear. Nevertheless, several reports of third body wear due to delamination and cohesive failure also show negative effects of the TiN-coating [3]. The hardness of TiN and TiNbN coatings are with 24 GPa much higher than oxidized zirconium and supports the findings for lower polyethylene wear and high scratch resistance.
The metal ion release analysis of TiN and TiNbN coatings during an immersion study showed a reduction of cobalt ions of 80% and 76%, respectively, compared to an uncoated CoCrMo implant [61]. Similarly, another immersion study found a reduction of 80% for cobalt, 63% for chromium and 48% for molybdenum ion using a TiNbN coated implant in comparison to its uncoated CoCrMo version [62].
4.3 Biotribology and biomechanics of the ZrN multilayer coating
4.3.1 Wear articulating against UHMWPE
Studies have shown a UHMWPE wear reduction of more than 50% (Figure 1) by using the ZrN multilayer coated femur and tibial components in comparison to their uncoated versions [17, 63]. A limitation of the standardized ISO 14243 test is that it only simulates the short-term performance of an implant, as it reproduces only about 3 years of in vivo service [64]. Based on in vivo measurements performed on eight patients with instrumented implants [65], a new highly demanding activities (HDA) knee wear simulation protocol was developed, which reproduces 15–30 years of in vivo service, depending on the activity level of the patient [66]. In the HDA wear protocol, the load and motion profiles of the high flexion activities of stairs ascending, stairs descending, chair raising, deep squatting and normal level walking are simulated. Moreover, the loading profiles were normalized to represent a patient weight of 100 kg. A wear test study performed with the HDA wear simulation protocol also demonstrated a 2.8-fold lower UHMWPE wear rate when articulated with ZrN multilayer coated implants compared to the uncoated version (Figure 1) [52].
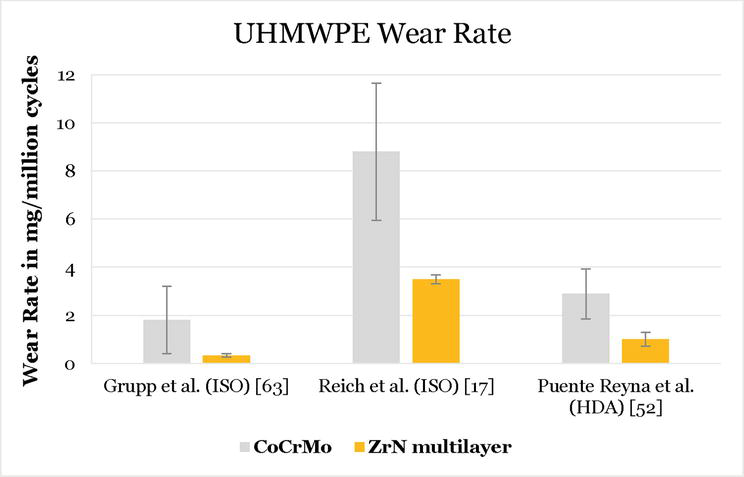
Figure 1.
UHMWPE wear rates comparison between uncoated CoCrMo and ZrN multilayer coated knee implants after ISO and HDA knee wear simulation protocols. Note: Reference [
Regarding the metal ion release, in vitro studies have shown that the ZrN multilayer coating (with a hardness of 25 GPa) is not impaired by failure modes such as delamination, surface disruption or flaking [17, 52, 53], even under third body particle contamination [17]. This has been confirmed by analysis of the metal ion concentration analysis performed on the test medium, where the metal ion release of the substrate material is several orders of magnitude lower than in the uncoated components (Figure 2). Moreover, it can be seen that the use of an HDA knee wear simulation increased the metal ion release in the uncoated components (both studies were performed on Columbus CR, Aesculap AG, Tuttlingen), whereas the ion release of the ZrN multilayer components remained in substantially a lower level.
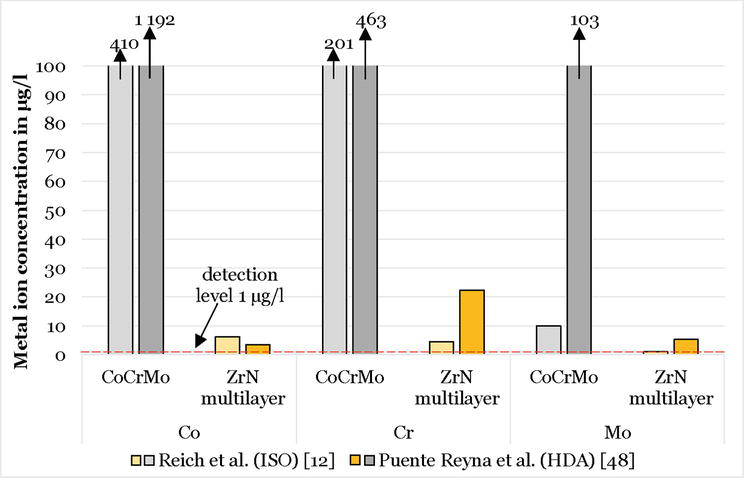
Figure 2.
Metal ion concentrations measured after 1 million cycles for the uncoated and ZrN coated version of Columbus CR/DD.
4.3.2 Wear against CFR-PEEK
Besides UHMWPE, the ZrN multilayer coating also articulates against carbon-fiber-reinforced poly-ether-ether-ketone (CFR-PEEK) components from a rotating hinged total knee prosthesis used in complex revision cases (EnduRo, Aesculap AG, Tuttlingen, Germany). The selection of CFR-PEEK as an articulation surface was selected, as it showed superior wear factors compared with polyethylene [67, 68] and favorable creep behavior. In an in vitro wear study, the ZrN multilayer coating was able to reduce the amount of CFR-PEEK wear particles produced from the flanges and flexion axis in comparison to the uncoated CoCrMo version of the implant by more than 90% [53]. A study on retrievals with an in vivo service between 12 and 60 months has confirmed the wear patterns on the CFR-PEEK components seen during the in vitro wear simulation test [69].
4.3.3 Bone cement fixation
The modified surface could result different behavior for cement fixation, which was, therefore, investigated. A study comparing the implant-cement-bone fixation strength between uncoated and ZrN multilayer coated tibial components by means of a push-out test and cementation in polyurethane blocks using different bone cement and cementation times was performed [70]. There were no statistical differences in the bone-cement fixation strength between the uncoated and the ZrN multilayer coated tibial components, and mixed failure modes at implant-cement and the cement-foam interface occurred.
Moreover, the fixation strength of the ZrN multilayer coated tibia components was statistically higher than that of a clinically long-term successful implant, whose failure mode was at the implant-cement interface.
4.3.4 Oxidation
Oxidation of the ZrN multilayer coating is normal behavior, as every metal in contact with biological systems will undergo a process of bio-corrosion [54, 71], and has been reported on in vitro studies before [52]. During the oxidation process, oxygen ions are exchanged with the nitrogen ions of the ZrN layer. This oxidation is visible in the ZrN layer because the color of zirconium alloys varies depending on the amount of oxygen and nitrogen, they contain [72, 73, 74, 75, 76]. It has, however, no influence on the biomechanical properties of the coating.
5. Clinical results and register data
The theoretical advantages of these improved implant surfaces need to be confirmed in patients. There are several clinical studies and several implants are monitored in arthroplasty registries. Because coated and standard implants are often used in different patient populations, a direct comparison of the implant performance is difficult. The more expensive implants with a surface modification or coating are more often used in patients with metal allergies. These patients have a high level of psychological distress when undergoing a TKA surgery [77], and it has been suggested that anxiety is the main reason for less favorable results in these patients [28]. It is therefore likely that patients with allergies to implant materials have a higher overall risk for revision because of an unsatisfactory result after TKA. This needs to be considered when looking at arthroplasty registry data.
5.1 Clinical studies
There are only a few studies comparing coated and standard implants. Table 1 summarizes studies with a minimum 5-year follow-up. If the performance of the implant is the outcome of interest, coated and standard implants should be tested in similar patient populations. As it is difficult to randomize patients with allergies against implant materials, in most studies only patients without known allergies were included. This is not the target population for hypoallergenic implants, but investigation of the mechanical properties of implants in these patients is reasonable.
| Author | Technology | Study design | Number of knees | follow-up (y) | Results |
|---|---|---|---|---|---|
| Lützner et al. 2022 [31] | ZrN | RCT ZrN vs. CoCrMo | 120 | 5 | No differences in cytokines |
| Postler et al. 2021 [78] | TiNbN | RCT TiNbN vs. CoCrMo | 118 | 5 | No difference in serum metal ion concentrations |
| Law et al. 2020 [79] | TNbN | Retrospective series TiNbN | 346 | 5 | 90% survival |
| Hauer et al. 2020 [80] | TiN | Retrospective series TiN cementless mobile vs. CoCrMo fixed cemented | 520 | 10.1 to | TAS was sign. Better for coated, KSF sign. Better for the uncoated group. |
| 14.9 | |||||
| Louwerens et al. 2020 [81] | TiN | RCT TiN cementless vs. CoCrMo | 101 | 10 | Survival uncoated 92% coated 94%. |
| Thomas et al. 2018 [32] | ZrN | Retrospective series ZrN vs. CoCrMo | 196 | 5 | Survival 98% coated vs. 97% uncoated; significant difference in proinflammatory cytokines with higher values in CoCrMo |
| Beyer et al. 2016 [82] | ZrN | RCT ZrN vs. CoCrMo | 120 | 5 | 100% survival of coated, 98.1% of uncoated group. |
| Hofer et al. 2014 [83] | OxZr | Retrospective series | 109 | 5.9 (5–10) | Mean KSS 92, mean KSF 81 |
| Innocenti et al. 2014 [84] | OxZr | Retrospective series | 98 | 11.3 (10–12.6) | Mean KSS 84, Mean KSF 83, 97.8% survival at 10Y. Two loosenings |
| Mohammend et al. 2014 [85] | TiN | Retrospective series TiN cementless | 305 | 6.5 (3–10) | 95.1% survival |
| Kim et al. 2012 [86] | OxZr | RCT in bilateral TKA OxZr vs. CoCrMo | 662 | 7.5 (6–8) | 100% survival at 7.5 Y in both groups |
| Hui et al. 2011 [87] | OxZr | RCT in bilateral TKA OxZr vs. CoCrMo | 80 | 5 | Mean KSS OxZr 89 vs. CoCrMo 92 |
Table 1.
RCT = randomized controlled trial; TAS = Tegner activity scale; KSS = knee society score; and KSF = knee society function score.
Studies are not available for all implants on the market. Published studies demonstrated no relevant differences between coated and standard TKA and overall good survival rates. Based on that data, modern coatings which have been tested in clinical studies seem to work well and there is no reason for concern. Because technologies are different, the published results cannot be transformed into different implants or coating technologies without additional clinical studies.
5.2 Arthroplasty registry results
The results of arthroplasty registries provide “real-world” information and are therefore an important source of data. However, it needs to be considered that, in most countries, patients who receive a coated TKA are somehow different from patients who receive a standard TKA, which results in patient groups with a different probability of revision. Therefore, as always with registry data, revision rates of an implant or implant group are not entirely be caused by the implant, but might also be influenced by the patient population.
There is a significant difference in the hazard ratio (HR) of revision between all the alternative surfaces (coatings and surface modifications) and standard CoCrMo femoral components in the Australian Arthroplasty Registry (AOANJRR). After 15 years, the CoCrMo implants have a revision rate of 6.3%, whereas the alternative surfaces have a revision rate of 9.4%. However, there are differences between the alternative surfaces. ZrN has lower revision rates than OxZr and TiN implants. After 5 years, the revision rate for the ZrN is 2.1% and for the uncoated components is 3.1%.
A review of more than 17,000 cases out of the AOANJRR evaluated outcomes of up to 12 years for the OxZr [88]. They found no significant difference for the 12-year cumulative percent revision (CPR) due to all causes (4.8% for CoCr and 7.7% for OxZr); non-septic causes, or osteolysis or loosening (0.6% for CoCr and 1.1% for OxZr). The only age-related difference was found with patients who were > 75 years old, for whom OxZr TKA had an increased CPR due to osteolysis or loosening.
In the National Joint Registry (NJR) of the United Kingdom, OxZr and ZrN have similar results in both versions [39]. AS Columbus (ZrN) had a cumulative revision rate of 2.42 compared to 2.05 of its uncoated version after 5 years. Genesis II Oxinium (OxZr) had a cumulative revision rate of 3.57 at 5 years and 7.67 at 15 years compared to the standard CoCr implants (2.05 at 5 years, 3.49 at 15 years). Both designs have a remarkably lower patient median age at primary TKA (AS Columbus 5 years lower, Genesis II Oxinium 12 years lower). The younger age in TKA is a higher risk for earlier revision, which could explain the slightly higher revision rates of both designs in the register [38].
Hypoallergenic implants demonstrated higher overall revision rates in the German Arthroplasty Registry after 3 years [89]. In this report, the main differences between coated and standard implant groups were the higher rate of metal allergies in female patients in the coated TKA group. Looking at the data in detail, it was recognized that for the most often used coated implants (Columbus, Vanguard, e.motion), there were no differences between coated and standard implants and that for these implants, revision rates were favorably lower than all implants. It seems that the higher revision rates are caused by less frequently used implants and that a general revision rate for “coated implants” is not sufficient. Each implant needs to be looked at separately.
An analysis of 62,177 primary TKAs from the Total Joint Replacement Registry in the USA compared OxZr to traditional CoCr TKA implants and showed no statistically significant higher risk for revision after a mean follow-up time of 2.8 years [90].
6. Practical approach for the use of coated implants
In all patients with suspected or confirmed allergy against implant materials, informed consent and shared decision-making are crucial to avoid negative psychological effects.
In primary TKA (Figure 3), all patients should be asked about allergies against implant materials before surgery. If an allergy is only self-reported, a test should be performed (usually a skin patch test). If the allergy is confirmed, pros and cons of different implants should be discussed with the patient, and a hypoallergenic implant should be considered. A multilayer-coated or oxidized zirconium implant is the best evaluated option in clinical studies and will probably have the best performance regarding the long-term reduction of metal ion release and wear.
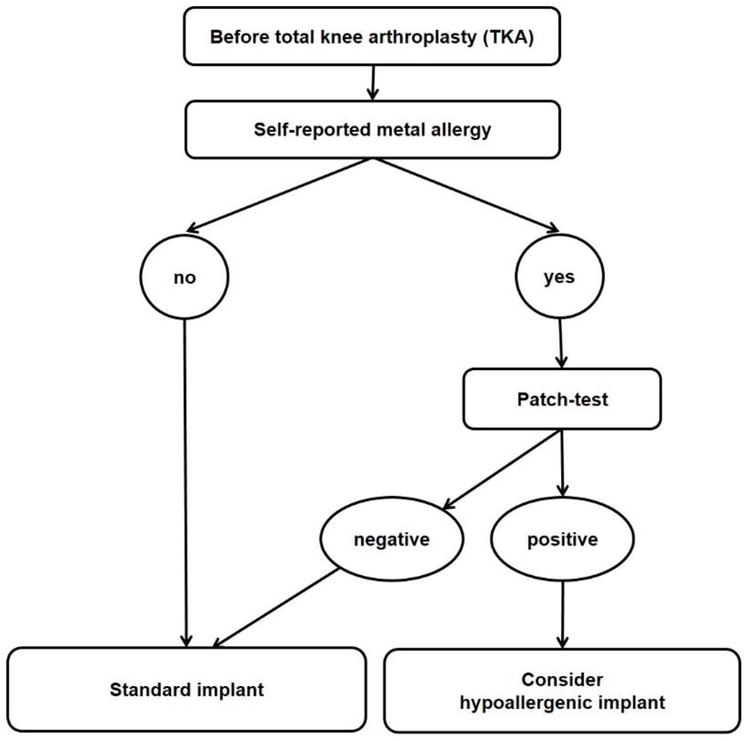
Figure 3.
A suggested algorithm for primary TKA [
In case of revision TKA (Figure 4) and suspected allergy against implant materials, additionally to the rule-out of other causes (especially periprosthetic joint infection), a histopathological evaluation (arthroscopic or open during two-stage revision) should be performed. If there is a confirmed allergy (Krenn type 6), a hypoallergenic implant should be considered as in primary TKA.
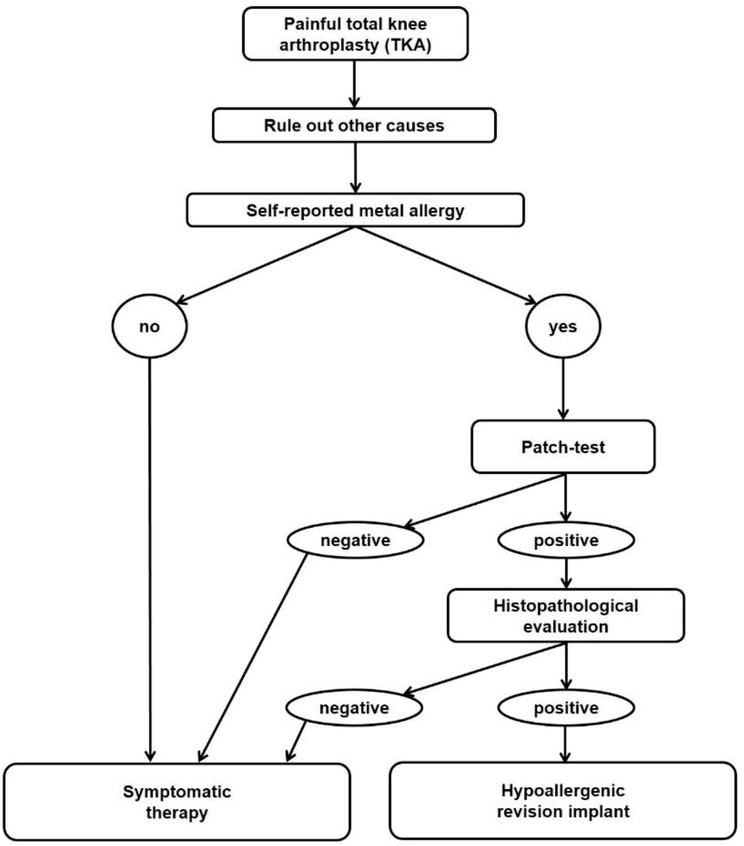
Figure 4.
A suggested algorithm for revision TKA [
7. Conclusion
Modern coatings are safe and provide in numerous pre-clinical studies advantages compared to standard implants regarding wear and metal ion release. They are, however, more expensive in many countries and can therefore often not be used routinely. Therefore, advantages and disadvantages need to be balanced to choose the patients who will benefit most. There is no general concern about metal ion release from CoCrMo standard implants for most patients. If patients present with a confirmed metal allergy, a multilayer-coated or oxidized zirconium implant is currently the best option for these patients.
Conflict of interest
Jörg Lützner has received research grants from Aesculap, Link, Mathys, Smith & Nepehw and ZimmerBiomet and honoraria for lectures from Aesculap, Mathys, and Pfizer. Ana Laura Puente Reyna, Brigitte Altermann and Thomas M. Grupp are employees of Aesculap AG, Tuttlingen, a manufacturer of orthopedic implants.
References
- 1.
Evans JT, Walker RW, Evans JP, Blom AW, Sayers A, Whitehouse MR. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. The Lancet. 2019; 393 :655-663. DOI: 10.1016/S0140-6736(18)32531-5 - 2.
Galetz MC, Fleischmann EW, Konrad CH, Schuetz A, Glatzel U. Abrasion resistance of oxidized zirconium in comparison with CoCrMo and titanium nitride coatings for artificial knee joints. Journal of Biomedical Materials Research. Part B, Applied Biomaterials. 2010; 93 :244-251. DOI: 10.1002/jbm.b.31581 - 3.
van Hove RP, Sierevelt IN, van Royen BJ, Nolte PA. Titanium-nitride coating of orthopaedic implants: A review of the literature. BioMed Research International. 2015; 2015 :485975. DOI: 10.1155/2015/485975 - 4.
Herbster M, Döring J, Nohava J, Lohmann CH, Halle T, Bertrand J. Retrieval study of commercially available knee implant coatings TiN, TiNbN and ZrN on TiAl6V4 and CoCr28Mo6. Journal of the Mechanical Behavior of Biomedical Materials. 2020; 112 :104034. DOI: 10.1016/j.jmbbm.2020.104034 - 5.
Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: Histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. Journal of Clinical Pathology. 2012; 65 :409-418. DOI: 10.1136/jclinpath-2011-200398 - 6.
Leyssens L, Vinck B, van der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology. 2017; 387 :43-56. DOI: 10.1016/j.tox.2017.05.015 - 7.
Christian WV, Oliver LD, Paustenbach DJ, Kreider ML, Finley BL. Toxicology-based cancer causation analysis of CoCr-containing hip implants: A quantitative assessment of genotoxicity and tumorigenicity studies. Journal of Applied Toxicology: JAT. 2014; 34 :939-967. DOI: 10.1002/jat.3039 - 8.
Kovochich M, Monnot A, Kougias DG, More SL, Wilsey JT, Qiu Q-Q , et al. Carcinogenic hazard assessment of cobalt-containing alloys in medical devices: Review of in vivo studies. Regulatory Toxicology and Pharmacology. 2021; 122 :104910. DOI: 10.1016/j.yrtph.2021.104910 - 9.
Lützner J, Günther K-P, Postler A, Morlock M. Metallionenfreisetzung nach Hüft- und Kniegelenkendoprothetik – Mechanismen, biologische Wirkungen und notwendige Diagnostik. [Metal Ion Release after Hip and Knee Arthroplasty—Causes, Biological Effects and Diagnostics]. Zeitschrift für Orthopädie und Unfallchirurgie. 2020; 158 :369-382. DOI: 10.1055/a-0929-8121 - 10.
Grupp TM, Baxmann M, Jansson V, Windhagen H, Heller KD, Morlock MM, Knaebel HP. How to proceed with asymptomatic modular dual taper hip stems in the case of acetabular revision. Materials (Basel). 2 Mar 2020; 13 (5):1098. DOI: 10.3390/ma1305109 - 11.
Bernstein DT, Meftah M, Paranilam J, Incavo SJ. Eighty-six percent failure rate of a modular-neck femoral stem design at 3 to 5 years: Lessons learned. The Journal of Bone and Joint Surgery. 2016; 98 :e49. DOI: 10.2106/JBJS.15.01082 - 12.
Dimitriou D, Liow MHL, Tsai T-Y, Leone WA, Li G, Kwon Y-M. Early outcomes of revision surgery for taper corrosion of dual taper total hip arthroplasty in 187 patients. The Journal of Arthroplasty. 2016; 31 :1549-1554. DOI: 10.1016/j.arth.2016.01.015 - 13.
Ghanem E, Ward DM, Robbins CE, Nandi S, Bono JV, Talmo CT. Corrosion and adverse local tissue reaction in one type of modular neck stem. The Journal of Arthroplasty. 2015; 30 :1787-1793. DOI: 10.1016/j.arth.2015.04.039 - 14.
Meftah M, Haleem AM, Burn MB, Smith KM, Incavo SJ. Early corrosion-related failure of the rejuvenate modular total hip replacement. The Journal of Bone and Joint Surgery. American Volume. 2014; 96 :481-487. DOI: 10.2106/JBJS.M.00979 - 15.
Gramlich Y, Hofmann L, Kress S, Ruckes C, Kemmerer M, Klug A, et al. Critically high metal ion levels found in metal-on-metal modular hinged knee arthroplasty: A comparison of two different systems. Bone Joint Journal. 2020; 104-B :376-385. DOI: 10.1302/0301-620X.104B3.BJJ-2021-0492.R2 - 16.
Kellens J, Berger P, Vandenneucker H. Metal wear debris generation in primary total knee arthroplasty: Is it an issue? Acta Orthopaedica Belgica. 2021; 87 :681-695. DOI: 10.52628/87.4.13 - 17.
Reich J, Hovy L, Lindenmaier H-L, Zeller R, Schwiesau J, Thomas P, et al. Preclinical evaluation of coated knee implants for allergic patients. Orthopade. 2010; 39 :495-502. DOI: 10.1007/s00132-009-1581-9 - 18.
Matar HE, Porter PJ, Porter ML. Metal allergy in primary and revision total knee arthroplasty: A scoping review and evidence-based practical approach. Bone Jt Open. 2021; 2 :785-795. DOI: 10.1302/2633-1462.210.BJO-2021-0098.R1 - 19.
Bravo D, Wagner ER, Larson DR, Davis MP, Pagnano MW, Sierra RJ. No increased risk of knee arthroplasty failure in patients with positive skin patch testing for metal hypersensitivity: A Matched Cohort study. The Journal of Arthroplasty. 2016; 31 :1717-1721. DOI: 10.1016/j.arth.2016.01.024 - 20.
Middleton STA. Allergy in total knee arthroplasty: A review of the facts. Bone Joint Journal. 2016; 98-B :437-441 - 21.
Guenther D, Thomas P, Kendoff D, Omar M, Gehrke T, Haasper C. Allergic reactions in arthroplasty: Myth or serious problem? International Orthopaedics. 2016; 40 :239-244. DOI: 10.1007/s00264-015-3001-6 - 22.
Münch HJ, Jacobsen SS, Olesen JT, Menné T, Søballe K, Johansen JD, et al. The association between metal allergy, total knee arthroplasty, and revision: Study based on the Danish knee arthroplasty register. Acta Orthopaedica. 2015; 86 :378-383. DOI: 10.3109/17453674.2014.999614 - 23.
Schäfer T, Böhler E, Ruhdorfer S, Weigl L, Wessner D, Filipiak B, et al. Epidemiology of contact allergy in adults. Allergy. 2001; 56 :1192-1196. DOI: 10.1034/j.1398-9995.2001.00086.x - 24.
Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: A systematic review. Journal of Bone and Joint Surgery. British Volume (London). 2012; 94 :1126-1134. DOI: 10.1302/0301-620X.94B8.28135 - 25.
Basko-Plluska JL, Thyssen JP, Schalock P. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011; 22 :65-79 - 26.
Thienpont E, Berger Y. No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: Case report and review of the literature. Knee Surgery, Sports Traumatology, Arthroscopy. 2013; 21 :636-640. DOI: 10.1007/s00167-012-2000-z - 27.
Nam D, Li K, Riegler V, Barrack RL. Patient-reported metal allergy: A risk factor for poor outcomes after total joint arthroplasty? The Journal of Arthroplasty. 2016; 31 :1910-1915. DOI: 10.1016/j.arth.2016.02.016 - 28.
Ferrer T, Hinarejos P, Goicoechea N, Leal-Blanquet J, Sanchez-Soler J, Torres-Claramunt R, et al. Anxiety is the cause of the worse outcomes of allergic patients after total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy. 2020; 28 :3135-3141. DOI: 10.1007/s00167-019-05780-0 - 29.
Shah K, Mohammed A, Patil S, McFadyen A, Meek RMD. Circulating cytokines after hip and knee arthroplasty: A preliminary study. Clinical Orthopaedics and Related Research. 2009; 467 :946-951. DOI: 10.1007/s11999-008-0562-3 - 30.
Langkilde A, Jakobsen TL, Bandholm TQ , Eugen-Olsen J, Blauenfeldt T, Petersen J, et al. Inflammation and post-operative recovery in patients undergoing total knee arthroplasty-secondary analysis of a randomized controlled trial. Osteoarthritis and Cartilage. 2017; 25 :1265-1273. DOI: 10.1016/j.joca.2017.03.008 - 31.
Lützner J, Beyer F, Lützner C, Thomas P, Summer B. Increased inflammatory response is associated with less favorable functional results 5 years after total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy. 11 Feb 2022. DOI: 10.1007/s00167-021-06836-w - 32.
Thomas P, Hisgen P, Kiefer H, Schmerwitz U, Ottersbach A, Albrecht D, et al. Blood cytokine pattern and clinical outcome in knee arthroplasty patients: Comparative analysis 5 years after standard versus “hypoallergenic” surface coated prosthesis implantation. Acta Orthopaedica. 2018; 89 :646-651. DOI: 10.1080/17453674.2018.1518802 - 33.
Spector BM, Ries MD, Bourne RB, Sauer WS, Long M, Hunter G. Wear performance of ultra-high molecular weight polyethylene on oxidized zirconium total knee femoral components. The Journal of Bone and Joint Surgery. American Volume. 2001; 83-A (Suppl. 2 Pt. 2):80-86. DOI: 10.2106/00004623-200100022-00004 - 34.
Kore L, Bates T, Mills G, Lybeck D. Oxidized zirconium total knee arthroplasty implant failure in a patient with knee instability. Arthroplast Today. 2020; 6 :552-555. DOI: 10.1016/j.artd.2020.06.015 - 35.
Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. The Lancet. 2017; 389 :1424-1430. DOI: 10.1016/S0140-6736(17)30059-4 - 36.
Civinini R, Carulli C, Matassi F, Lepri AC, Sirleo L, Innocenti M. The survival of total knee arthroplasty: Current data from registries on tribology: Review Article. HSS Journal. 2017; 13 :28-31. DOI: 10.1007/s11420-016-9513-9 - 37.
Pabinger C, Berghold A, Boehler N, Labek G. Revision rates after knee replacement. Cumulative results from worldwide clinical studies versus joint registers. Osteoarthritis and Cartilage. 2013; 21 :263-268. DOI: 10.1016/j.joca.2012.11.014 - 38.
Graves S, de Steiger R, Lewis P, Harris I. Australian orthopaedic association national joint replacement registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty. 2021:186-286 - 39.
Reed M, Brittain R, Howard P, Lawrence S, Stonadge J, Wilkinson M, Wilton T. National Joint Registry England, Wales, Northern Ireland & Isle of Man. 18th Annual Report 2021. 2021: 135-212 - 40.
Long WJ, Bryce CD, Hollenbeak CS, Benner RW, Scott WN. Total knee replacement in young, active patients: Long-term follow-up and functional outcome: A concise follow-up of a previous report. The Journal of Bone and Joint Surgery. 2014; 96 :e159. DOI: 10.2106/JBJS.M.01259 - 41.
Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bulletin of the NYU Hospital for Joint Diseases. 2009; 67 :182-188 - 42.
Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005; 26 :1271-1286. DOI: 10.1016/j.biomaterials.2004.04.035 - 43.
Catelas I, Wimmer MA, Utzschneider S. Polyethylene and metal wear particles: Characteristics and biological effects. Seminars in Immunopathology. 2011; 33 :257-271. DOI: 10.1007/s00281-011-0242-3 - 44.
Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. Journal of Biomedical Materials Research. Part A. 2009; 91 :1252-1262. DOI: 10.1002/jbm.a.32625 - 45.
Magone K, Luckenbill D, Goswami T. Metal ions as inflammatory initiators of osteolysis. Archives of Orthopaedic and Trauma Surgery. 2015; 135 :683-695. DOI: 10.1007/s00402-015-2196-8 - 46.
Ackerman IN, Busija L, Lorimer M, de Steiger R, Graves SE. Monitoring the lifetime risk of revision knee arthroplasty over a decade: A population-level analysis of Australian national registry data. Bone Joint Journal. 2022; 104-B :613-619. DOI: 10.1302/0301-620X.104B5.BJJ-2021-1219.R1 - 47.
Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb W-C. Serum metal ion exposure after total knee arthroplasty. Clinical Orthopaedics and Related Research. 2007; 461 :136-142. DOI: 10.1097/BLO.0b013e31806450ef - 48.
Thomas P, Weik T, Roider G, Summer B, Thomsen M. Influence of surface coating on metal ion release: Evaluation in patients with metal allergy. Orthopedics. 2016; 39 :S24-S30. DOI: 10.3928/01477447-20160509-08 - 49.
Thomsen M, Rozak M, Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: Disappearance of symptoms after revision with a special surface-coated TKA—A case report. Acta Orthopaedica. 2011; 82 :386-388. DOI: 10.3109/17453674.2011.579521 - 50.
Mitchelson AJ, Wilson CJ, Mihalko WM, Grupp TM, Manning BT, Dennis DA, et al. Biomaterial hypersensitivity: Is it real? Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. BioMed Research International. 2015; 2015 :137287. DOI: 10.1155/2015/137287 - 51.
Roy ME, Whiteside LA, Ly KK, Gauvain MJ. Cobalt-chromium femoral components developed scratches and released metal debris in simulated wear whereas ceramic femoral components did not. Bone Joint J. 2021; 103 :94-101 - 52.
Puente Reyna AL, Fritz B, Schwiesau J, Schilling C, Summer B, Thomas P, et al. Metal ion release barrier function and biotribological evaluation of a zirconium nitride multilayer coated knee implant under highly demanding activities wear simulation. Journal of Biomechanics. 2018; 79 :88-96. DOI: 10.1016/j.jbiomech.2018.07.043 - 53.
Grupp TM, Giurea A, Miehlke RK, Hintner M, Gaisser M, Schilling C, et al. Biotribology of a new bearing material combination in a rotating hinge knee articulation. Acta Biomaterialia. 2013; 9 :7054-7063. DOI: 10.1016/j.actbio.2013.02.030 - 54.
Kretzer JP, Reinders J, Sonntag R, Hagmann S, Streit M, Jeager S, et al. Wear in total knee arthroplasty-just a question of polyethylene? Metal ion release in total knee arthroplasty. International Orthopaedics (SICOT). 2014; 38 :335-340. DOI: 10.1007/s00264-013-2162-4 - 55.
Malikian R, Maruthainar K, Stammers J, Cannon SR, Carrington R, Skinner JA, et al. In vivo roughening of retrieved total knee arthroplasty femoral components. The Knee. 2014; 21 :278-282. DOI: 10.1016/j.knee.2012.09.008 - 56.
Davidson JA. Characteristics of metal and ceramic total hip bearing surfaces and their effect on long-term ultra high molecular weight polyethylene wear. Clinical Orthopaedics and Related Research. 1993; 294 :361-378 - 57.
Paschoal AL, Vanancio EC, Canale LC, da Silva OL, Huerta-Vilca D, Motheo AJ. Metallic biomaterials TiN-coated: Corrosion analysis and biocompatibility. Artificial Organs. 2003; 27 :461-464. DOI: 10.1046/j.1525-1594.2003.07241.x - 58.
Pilz M, Staats K, Tobudic S, Assadian O, Presterl E, Windhager R, et al. Zirconium nitride coating reduced staphylococcus epidermidis biofilm formation on orthopaedic implant surfaces: An In Vitro Study. Clinical Orthopaedics and Related Research. 2019; 477 :461-466. DOI: 10.1097/CORR.0000000000000568 - 59.
Schüttler KF, Efe T, Heyse TJ, Haas SB. Oxidized zirconium bearing surfaces in total knee arthroplasty: Lessons learned. The Journal of Knee Surgery. 2015; 28 :376-381. DOI: 10.1055/s-0035-1551836 - 60.
Lee JKL, Maruthainar K, Wardle N, Haddad F, Blunn GW. Increased force simulator wear testing of a zirconium oxide total knee arthroplasty. The Knee. 2009; 16 :269-274. DOI: 10.1016/j.knee.2008.12.010 - 61.
Bader R, Bergschmidt P, Fritsche A, Ansorge S, Thomas P, Mittelmeier W. Alternative Werkstoffe und Lösungen in der Knieendoprothetik für Patienten mit Metallallergie. [Alternative materials and solutions in total knee arthroplasty for patients with metal allergy]. Der Orthopäde. 2008; 37 :136-142. DOI: 10.1007/s00132-007-1189-x - 62.
Ragone V, Canciani E, Biffi CA, D’Ambrosi R, Sanvito R, Dellavia C, et al. CoCrMo alloys ions release behavior by TiNbN coating: An in vitro study. Biomedical Microdevices. 2019; 21 :61. DOI: 10.1007/s10544-019-0417-6 - 63.
Grupp TM, Schroeder C, Kyun Kim T, Miehlke RK, Fritz B, Jansson V, et al. Biotribology of a mobile bearing posterior stabilised knee design—Effect of motion restraint on wear, tibio-femoral kinematics and particles. Journal of Biomechanics. 2014; 47 :2415-2423. DOI: 10.1016/j.jbiomech.2014.04.020 - 64.
Schwiesau J, Schilling C, Kaddick C, Utzschneider S, Jansson V, Fritz B, et al. Definition and evaluation of testing scenarios for knee wear simulation under conditions of highly demanding daily activities. Medical Engineering & Physics. 2013; 35 :591-600. DOI: 10.1016/j.medengphy.2012.07.003 - 65.
Bergmann G, Bender A, Graichen F, Dymke J, Rohlmann A, Trepczynski A, et al. Standardized loads acting in knee implants. PLoS One. 2014; 9 :e86035. DOI: 10.1371/journal.pone.0086035 - 66.
Schwiesau J, Fritz B, Kutzner I, Bergmann G, Grupp TM. CR TKA UHMWPE wear tested after artificial aging of the vitamin E treated gliding component by simulating daily patient activities. BioMed Research International. 2014; 2014 :567374. DOI: 10.1155/2014/567374 - 67.
Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007; 28 :4845-4869. DOI: 10.1016/j.biomaterials.2007.07.013 - 68.
Scholes SC, Unsworth A. Wear studies on the likely performance of CFR-PEEK/CoCrMo for use as artificial joint bearing materials. Journal of Materials Science: Materials in Medicine. 2009; 20 :163-170. DOI: 10.1007/s10856-008-3558-3 - 69.
Schierjott RA, Giurea A, Neuhaus HJ, Schwiesau J, Pfaff AM, Utzschneider S, Tozzi G, Grupp TM. Analysis of Carbon Fiber Reinforced PEEK Hinge Mechanism Articulation Components in a Rotating Hinge Knee Design: A Comparison of In Vitro and Retrieval Findings. Biomed Research International. 2016; 2016 :7032830. DOI: 10.1155/2016/7032830 - 70.
Grupp TM, Schilling C, Schwiesau J, Pfaff A, Altermann B, Mihalko WM. Tibial implant fixation behavior in total knee arthroplasty: A study with five different bone cements. The Journal of Arthroplasty. 2020; 35 :579-587. DOI: 10.1016/j.arth.2019.09.019 - 71.
Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. The Journal of Bone and Joint Surgery. 1998; 80 :268-282. DOI: 10.2106/00004623-199802000-00015 - 72.
Brown R, Alias MN, Fontana R. Effect of composition and thickness on corrosion behavior of TiN and ZrN thin films. Surface and Coating Technology. 1993; 62 :467-473. DOI: 10.1016/0257-8972(93)90285-V - 73.
Cheng Y, Zheng YF. A study of ZrN/Zr coatings deposited on NiTi alloy by PIIID technique. IEEE Transactions on Plasma Science. 2006; 34 :1105-1108. DOI: 10.1109/TPS.2006.877502 - 74.
Ferreira SC, Ariza E, Rocha LA, Gomes JR, Carvalho P, Vaz F, et al. Tribocorrosion behaviour of ZrOxNy thin films for decorative applications. Surface and Coatings Technology. 2006; 200 :6634-6639 - 75.
Milošev I, Strehblow H-H, Navinseka B. Comparison of TiN, ZrN and CrN hard nitride coatings: Electrochemical and thermal oxidation. Thin Solid Films. 1997; 303 :246-254. DOI: 10.1016/S0040-6090(97)00069-2 - 76.
Reddy G, Ramana JV, Kumar S, Kumar SV, Raju VS. Investigations on the oxidation of zirconium nitride films in air by nuclear reaction analysis and backscattering spectrometry. Applied Surface Science. 2007; 253 :7230-7237. DOI: 10.1016/j.apsusc.2007.03.004 - 77.
Peña P, Ortega MA, Buján J, De la Torre B. Influence of Psychological Distress in Patients with hypoallergenic total knee arthroplasty. Treatment algorithm for patients with metal allergy and knee osteoarthritis. International Journal of Environmental Research and Public Health. 3 Jun 2021; 18 (11):5997. DOI: 10.3390/ijerph18115997 - 78.
Postler A, Beyer F, Lützner C, Tille E, Lützner J. Die Anwendung antiallergisch beschichteter Knieendoprothesen ist mittelfristig sicher: Eine randomisierte kontrollierte Studie [The use of knee prostheses with a hypoallergenic coating is safe in the medium term: A randomized controlled study]. Orthopade (German). 3 Nov 2021. DOI: 10.1007/s00132-021-04186-6 - 79.
Law JI, Morris MJ, Hurst JM, Berend KR, Lombardi AV, Crawford DA. Early outcomes of an alternative bearing surface in primary total knee arthroplasty in patients with self-reported metal allergy. Arthroplast Today. 2020; 6 :639-643. DOI: 10.1016/j.artd.2020.07.021 - 80.
Hauer G, Leitner L, Ackerl MC, Klim S, Vielgut I, Ehall R, et al. Titanium-nitride coating does not result in a better clinical outcome compared to conventional cobalt-chromium Total Knee Arthroplasty after a Long-Term Follow-Up: A Propensity Score Matching Analysis. Coatings. 2020; 10 :442. DOI: 10.3390/coatings10050442 - 81.
Louwerens JKG, Hockers N, Achten G, Sierevelt IN, Nolte PA, van Hove RP. No clinical difference between TiN-coated versus uncoated cementless CoCrMo mobile-bearing total knee arthroplasty; 10-year follow-up of a randomized controlled trial. Knee Surgery, Sports Traumatology, Arthroscopy. 2021; 29 :750-756. DOI: 10.1007/s00167-020-05997-4 - 82.
Beyer F, Lützner C, Kirschner S, Lützner J. Midterm results after coated and uncoated TKA: A randomized controlled study. Orthopedics. 2016; 39 :S13-S17. DOI: 10.3928/01477447-20160509-10 - 83.
Hofer JK, Ezzet KA. A minimum 5-year follow-up of an oxidized zirconium femoral prosthesis used for total knee arthroplasty. The Knee. 2014; 21 :168-171. DOI: 10.1016/j.knee.2013.08.015 - 84.
Innocenti M, Carulli C, Matassi F, Carossino AM, Brandi ML, Civinini R. Total knee arthroplasty in patients with hypersensitivity to metals. International Orthopaedics. 2014; 38 :329-333. DOI: 10.1007/s00264-013-2229-2 - 85.
Mohammed A, Metcalfe A, Woodnutt D. Medium-term outcome of titanium nitride, mobile bearing total knee replacement. Acta Orthopaedica Belgica. Jun 2014; 80 :269-275 - 86.
Kim YH, Park JW, Kim JS. Comparison of the Genesis II total knee replacement with oxidised zirconium and cobalt-chromium femoral components in the same patients: A prospective, double-blind, randomised controlled study. Journal of Bone and Joint Surgery. British Volume (London). 2012; 94 :1221-1227 - 87.
Hui C, Salmon L, Maeno S, Roe J, Walsh W, Pinczewski L. Five-year comparison of oxidized zirconium and cobalt-chromium femoral components in total knee arthroplasty: A randomized controlled trial. The Journal of Bone and Joint Surgery. 2011; 93 :624-630. DOI: 10.2106/JBJS.I.01753 - 88.
Vertullo CJ, Lewis PL, Graves S, Kelly L, Lorimer M, Myers P. Twelve-year outcomes of an oxinium total knee replacement compared with the same cobalt-chromium design: An analysis of 17,577 prostheses from the australian orthopaedic association national joint replacement registry. The Journal of Bone and Joint Surgery. American Volume. 2017; 99 :275-283. DOI: 10.2106/JBJS.16.00092 - 89.
Grimberg AW, Grupp TM, Elliott J, Melsheimer O, Jansson V, Steinbrück A. Ceramic coating in cemented primary total knee arthroplasty is not associated with decreased risk of revision due to early prosthetic joint infection. The Journal of Arthroplasty. 2021; 36 :991-997. DOI: 10.1016/j.arth.2020.09.011 - 90.
Inacio MCS, Cafri G, Paxton EW, Kurtz SM, Namba RS. Alternative bearings in total knee arthroplasty: Risk of early revision compared to traditional bearings: an analysis of 62,177 primary cases. Acta Orthopaedica. 2013; 84 :145-152. DOI: 10.3109/17453674.2013.784660