Abstract
Total hip arthroplasty (THA) stands as a reliable and effective way to manage end-stage hip disease secondary to a number of aetiologic conditions. While target ‘safe zones’ are widely quoted and endorsed, an increasingly robust body of evidence suggests that such idealised implantation goals have limited utility in patient-to-patient considerations and that even with a precise goal in mind, surgeons perform inconsistently in achieving these targets intra-operatively. Inter-patient variability, the concept of ‘functional’ safe zones and the largely under-appreciated impact of poor patient positioning (and progressive loss of position during the case) are all recognised and evidence-supported opponents of conventional ‘40/15’ approaches. In an environment whereby accountable cost utility, maximised surgical consistency (i.e., outlier minimisation), improved attainment of target position, and awareness of the radiation exposure burden of many pre-operative templating regimes are all paramount, there appears to be an increasing role for the application of imageless ‘mini’ intra-operative navigation systems for primary (and revision) THA procedures. This chapter reviews the evolution of THA navigation and discusses contemporary applications, defines the challenges associated with unanticipated pelvic movement, and explores potential future directions in the use of this exciting technology.
Keywords
- technology-assisted surgery
- hip navigation
- computer navigation
- THA
- total hip replacement
1. Introduction
Total hip replacement remains a tried-and-true method for managing hip pain and dysfunction resultant from end-stage degenerative disease and a number of other medical conditions [1, 2, 3]. It has a long-standing proven clinical track record with strong evidence to support consistent improvements in patient function and satisfaction—indeed primary total hip arthroplasty (THA) has been claimed as one of the most significant surgical advances of the 20th century [4].
In the late 1970s, Lewinnek and colleagues generated the landmark paper proposing the acetabular ‘safe zone’—an idealised target orientation for component placement—suggested to be associated with decreased risk of prosthetic dislocation [5]. Nearly 45 years later, the paper stands as one of the most cited in the orthopaedic literature [6]. The ‘40/15 safe zone’ of Lewinnek (inferring a target acetabular component insertion position of 40° of abduction and 15° of anteversion, each +/− 10°) (Figure 1) has largely become the ‘ideal’ cup orientation for hip replacement surgeons and forms the basis of most conventional implantation tools/aids. This fundamental premise of THA surgery has however been challenged extensively in the contemporary literature with many authors suggesting limited value for these targets on a patient-by-patient level—several larger, reputable, papers have shown large proportions of post-operative dislocations occurring well within the defined ‘safe zone’ [7, 8]. The suggestion that ‘one size does
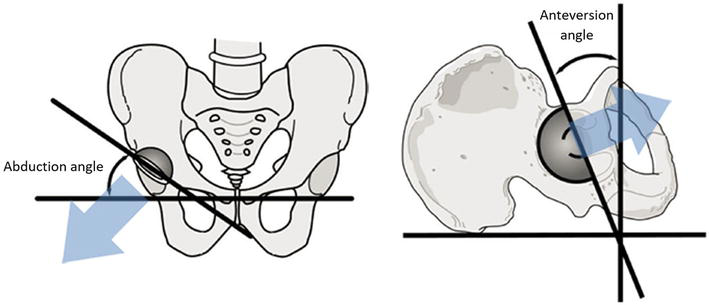
Figure 1.
Pelvic abduction angle (also known as ‘inclination’ or ‘opening’ angle) and anteversion angle determination.
Acetabular prosthesis implantation angles have been shown to affect peri-articular muscle mechanical advantage, rates of dislocation, gait and gait efficiency, limb lengths, impingement, noise generation, loosening, postoperative range-of-movement, liner wear and overall revision rates [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Balanced biomechanical and anatomical reconstruction of the joint is therefore critical to achieve function, enduring longevity and prevention of avoidable complications following surgery [15, 16, 20]. Dislocation rates following primary THA are acknowledged to occur in 1–4% of cases, with ‘instability’ accounting for approximately 23% of all revisions and remains the most common reason for such surgery in the United States [24, 25]. Preoperative templating from a plain anterior-posterior radiograph is the primary method for initial evaluation and forms the cornerstone of pre-operative prosthesis position planning, however the value of such images are subject to degradation due to uncompensated patient pelvic malposition. Suboptimal acetabular component position can significantly negatively impact the results of a hip arthroplasty, including increased risk of instability, impingement, dislocation and cup failure [12, 14, 16, 19, 26, 27, 28, 29, 30]. Correct template positioning influences the accuracy of acetabular cup placement planning and hence the long-term success of the THA.
Traditional freehand THA techniques rely heavily upon surgeon judgement to manually place acetabular components accurately. Computer navigation to reduce acetabular malpositioning has been used for more than 20 years, demonstrating improved attainment of target cup placement and variable reports of improvement in clinical outcomes, including reducing rates of revision [31, 32, 33]. By comparison, the prestigious Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) began collecting data on the use of computer-navigated total
Historically two separate means of informing computer-assisted navigation systems have existed—those reliant upon pre-operative imaging and ‘imageless’ systems. Although plain X-ray-based renditions do exist [37], most ‘image driven’ systems rely on images generated from pre-operatively obtained computer tomography (CT) scans using proprietary image reconstruction and feature recognition. Common to ‘imageless’ systems is some method of anatomic feature recognition determined during the surgery itself which informs the surgical navigation plan. The use of CT-based navigation has been shown to be highly accurate, however it is burdened by the associated cost, the need for dedicated pre-operative imaging, and incumbent radiation exposure risk—all of which have been linked to low levels of clinical utilisation [38, 39, 40, 41].
Imageless intra-operative navigation systems allow real-time, surgeon-controlled, determination of leg length and offset changes, and three-dimensional (3D) cup position [42]. The key features of these commercially-available systems are intended to overcome some of the recognised barriers to uptake associated with ‘imaging-based’ navigation, and are already in widespread use [3]. This chapter aims to review the rationale for, evolution of, and current evidence base supporting the use of such ‘imageless’ navigation tools and also provide understanding as to why pelvic positional variability makes such systems of high value, as well as exploring some of the exciting cutting-edge extensions of such technology into the foreseeable future.
2. Recognising the importance of optimised acetabular component orientation
Optimal insertional orientation of the acetabular component during THA is a critical determinant of many tangible outcomes, including construct stability [43]. At its extreme, malpositioning may lead to prosthetic dislocation. With the majority of THAs currently still being performed in a lateral decubitus patient position [43, 44], factors which introduce inconsistency or error in achieving the desired final cup position have been extensively explored. Sound previous research has confirmed the following: 1. there is great inconsistency and often poor reproducibility in the accuracy with which a true decubitus position is achieved during the ‘set up’ phase of a THA operation; 2. conventional positioning devices perform poorly in maintaining the initial set up position during the performance of a THA; 3. there is considerable patient loss-of-position during the operation itself (i.e. the position of the pelvis changes during surgery); 4. an erroneous pelvic position (from the start of the operation) and/or a loss of position during the procedure introduces a substantial potential for error in the ultimate insertional orientation of the cup; 5. suboptimal cup position has been strongly associated with a number of poor outcome measures, including wear, increased revision rate and dislocation.
A number of previous investigations have attempted to quantify the ‘average’ amount of unintended pelvic movement which occurs during the performance of a routine primary THA. These results are discussed in more detail later herein. Interpretation of such information has however—in many instances—been clouded by inconsistent data collection methods or by unreliable measurement approaches.
3. Understanding the relationship between pelvic movement and resultant acetabular component position
While most surgeons agree that accurate implantation of the acetabular component of a THA is important for patient outcomes, the ‘ideal’ position is far from universally agreed upon [44, 45]. While the historic reference of Lewinnek’s safe zone has formed the basis of most ‘target’ cup positions [46, 47], many contemporary authors suggest that there may be merit in a ‘patient-specific’ orientation goal and that ‘one size’ does not fit all [10, 11]. Indeed, many proponents advocate for individualised patient assessment—often in the form of pre-operative functional imaging [48, 49, 50]—to inform intra-operative decision making. Deviation from an ‘anatomically neutral’ starting position can have considerable negative impact on resultant cup insertion decision making. One key determinant ‘pelvic tilt’ (or ‘roll’) reflects the divergent angle between the anterior pelvic plane (APP) and a vertical line in the anatomical (standing) position [51] (Figure 2). The large recent study of 1517 THAs by Pierrepont and colleagues suggested that in nearly 20% of patients the extent of functional sagittal pelvic rotation (reflecting pelvic tilt) identified could potentially lead to construct instability using historical ‘safe zone’ targets [50]. This is a staggeringly high proportion.
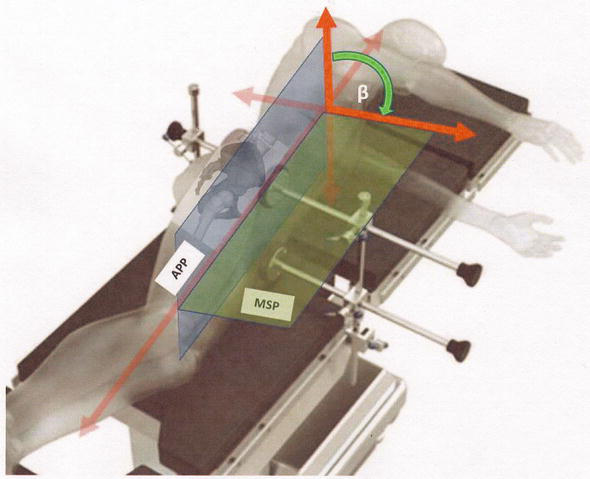
Figure 2.
Pelvic tilt. Measurement of the anterior tilt angle in a lateral decubitus position. Forward tilt is determined as the angle subtended by the difference in degrees from the true APP (i.e. the vertical starting position) to the measured APP as it approaches the MSP (i.e. as the pelvis rolls anteriorly) [
The effects of changing pelvic position on pelvic tilt has been well studied [52, 53, 54]. When the pelvis tilts posteriorly during basic pre-operative functional screening, the respective anteversion and abduction angles of the final acetabular implant position increases, which may in turn lead to excessive wear due to neck impingement and edge loading, with an increased risk of dislocation [19]. Prediction of pelvic displacement before surgery has key importance for accurate placement of the acetabular implant despite historically being an under-valued consideration. Objectively, Babisch et al. demonstrated that acetabular cup positions are affected by pelvic tilt on CT models, with good accuracy and reproducibility [55]. Similarly, a previous study by Maratt et al. using a computer-generated 3D model also demonstrated the substantial effect of pelvic tilt on resultant acetabular angles [56]. In a practical sense, the functional angle of the acetabular implant is directly related to the pelvic tilt angle, with the anteversion angle of the acetabular implant changing by approximately 0.7° with every degree of change in pelvic inclination [43]. Therefore, only small linear magnitude changes can significantly affect endpoint cup version and contribute to construct instability.
In contrast, until recently there has been a lack of literature describing the isolated effects of radiographic pelvic rotation (PR) on preoperative acetabular planning angulation of acetabular prostheses. The recent work of Lourens et al. used high resolution 3D CT pelvis models generated from healthy controls and arthroplasty patients to quantify the effects of pelvic rotation on acetabular cup position in various static planes simulating radiographic errors in basic imaging used for component templating [2]. They concluded that pelvic rotation can also significantly impact on the perceived acetabular angles observed on an AP pelvic radiograph used for pre-operative planning, which can in turn result in poor prosthetic placement and subsequent poorer long-term clinical outcomes. Supportive of the reliability of conventional approaches, the presented data indicated that PR of less than 20° however was unlikely to have a clinical impact of preoperative measurements and therefore may serve as a guide for clinical application and operative planning.
4. Pre-operative assessment: setting a target position
Respecting that the optimal prosthetic position for an acetabular component is likely to be subtly (or not so subtly) different for each individual patient, establishing a clear target position for the acetabular component of a THA is of critical importance. Even once a ‘target’ is defined, attainment of this can be a challenging process. As discussed later herein, inaccurate patient set up, loss of pelvic position during the procedure (i.e. patient movement) and errors in intended implantation angles can all undermine the achieved outcome [2, 43]. Langston et al. [57] suggested that a change in pelvic tilt of 13° or more on pre-operative assessment may be deemed unfavourable as this will result in a change in the functional anteversion of the acetabulum of 10°. This has the potential to place even a well-orientated component outside of a +/−10° target safe zone [57]. In the same work, the authors suggested that unfavourable pelvic mobility was independently associated with limited lumbar flexion, a more posterior standing pelvic tilt and increasing age [57, 58]. Unsurprisingly, they strongly advocated for pre-operative functional X-ray imaging [57]. It is noteworthy that none of the three associated factors they determined are immediately amendable to peri-operative correction prior to elective THA and may also thus be considered immutable (albeit important perhaps to recognise and consider). In extreme such cases, there is already a trend for some surgeons to move towards large head and/or dual mobility bearings in an attempt to increase the functional safe range-of-movement [59] in instances whereby concerns regarding spinopelvic stiffness have been raised. Even with such informed pre-surgical patient data, what best to ‘do’ with this information is less clear. Simply centring the implanted cup to the middle of the functional movement range has inherent risk and may not necessarily result in the perfect construct orientation to accommodate the rigours of daily activity. Recognising that final construct stability is a composite of optimised component mechanics AND the concurrent effect of the static and dynamic elements of the surrounding soft tissue envelope is a far too often under-acknowledged reality.
The process of high precision data capture with pre-operative functional imaging is also not without its challenges. True lateral pelvic X-rays (required for accurate conventional angular measurement) can be technically challenging to obtain and—under present conditions—at best reflect a series of pre-determined static captures of the bony relationship between the lower lumbosacral spine, pelvis and proximal femur [43]. These are not dynamic measures and do not directly take into account the critical impact of the surrounding soft tissue envelopes. Using the more commonly employed proprietary functional x-ray series, the relationship of the key bony elements in the extremes of motion are not represented—likely the positions most vulnerable to permit prosthetic dislocation [43].
Undoubtedly, an awareness of spinopelvic movement parameters also allows informed consideration of customised/patient-specific cup implantation targets [50, 60, 61, 62, 63]. Many centres now incorporate pre-operative spinopelvic movement assessment into routine work up pathways [64]. While it is clear that fundamental clinical assessment alone is insufficient to fully appreciate the linked movement characteristics of the human spine and pelvis on a patient-by-patient basis [64], how best to interpret often complex pre-operative imaging data and how to best apply this information to target cup planning [51] remains unclear and represents an opportunity for future investigation.
Recent work has suggested potential enhanced value with pre-operative simultaneous biplanar imaging [37, 64], as compared to conventional plain film X-rays. The proprietary EOS imaging system (Euronext: EOSI; Paris, France) is touted to reduce the radiation dose by two thirds as compared to equivalent plain X-ray imaging [64, 65]. Such technologies permit simultaneous capture of precisely orthogonal X-ray images in an upright, physiological load-bearing position and are claimed to be more accurate and less dependent on patient positioning [64]. Given that some have suggested limited practical utility of plain film X-rays in judging sagittal pelvic tilt [66] consideration of EOS (or other high precision imaging modalities) may hold merit. However, while the current science may suggest a role for EOS (or EOS-like imaging means) in replacing pre-operative radiographic assessment [64], the technology is not universally available and carries associated expense [43].
5. Intra-operative execution of the surgical plan
During the THA operation itself, three considerations become important with respect to the accuracy of definitive cup placement. Firstly, is the original position of the patient (as a surrogate for the true pelvis position). In the lateral (i.e. decubitus) set up, the surgeon/surgical team endeavour to ensure the patient’s pelvic sagittal plane (PSP) is horizontally orientated. In most practical senses, this refers to this key alignment plane being parallel to the theatre floor [67, 68]. Classically, surgeons have relied on palpation of key bony landmarks (i.e. ASIS and pubic symphysis) [69, 70, 71] to determine if the vertically-orientated APP [72] is indeed perpendicular to the flat level surface of the operating table (Figure 2). Direct and accurate localisation of the contralateral ASIS for APP determination can be challenging in the lateral position [72], especially with increasing BMI. Unsurprisingly, there is considerable inaccuracy in this subjective process [73] which assumes both landmark symmetry and an ability of the surgeon to accurately appreciate the location of such landmarks. An array of commonly-used positioning aids are employed for achieving and maintaining the true lateral orientation for THA. These usually involve some combination of posterior sacral block [74] and an anterior symphyseal bolster or ASIS post [74]—the latter of which may involve single or paired extensions. More proprietary universal lateral positioners [75] or peg boards [76] are also used with reasonable quoted effect. Interestingly, in a 2019 UK nationwide investigation however, Rutherford and colleagues explored surgeons’ sentiment towards current positioning tools [77]. More than 35% of respondents were ‘unhappy’ with their current supports, while less than a third (31%) felt their current positioning supports were rigid and reliably stable [77]. The need for better positioning tools and supports is almost unanimously championed [74, 76].
Previous authors have proposed customised pelvic orientation devices for use during initial set up claiming simplicity of use and improved accuracy and reproducibility in achieving a pelvis horizontal in the sagittal plane [67]. To date, despite the potential value of such positioning aids, they have failed to attract mainstream uptake and use. Iwakiri and colleagues [78] reported a custom variation of an existing positioning device with the addition of an extra compression pad [78]. Described as ‘simple, minimally invasive and cost effective’ [78] the authors were able to show significant reductions in intra-operative sagittal pelvic tilt. Other studies have shown highly significant differences (p < 0.001) in the maintenance of pelvic position during surgery when comparing the type of mechanical support used [76].
Beyond blaming the tools, multiple studies have shown significant variation in the ability of surgeons/surgical teams to accurately position the pelvis for THA surgery [76]. The 2011 work of Nishikubo and colleagues used in-theatre fluoroscopy to check for pelvic positioning errors prior to commencement of surgery [79]. With a pelvis orientated with 0° of tilt versus the horizontal sagittal plane as the target standard, they reported a mean positioning error of nearly 6°—this before the operation had even begun [79]. The later study of Lambers et al. reported a more modest error of closer to 3° but a wider range of recorded starting errors as high as 13° [80]. In a subanalysis the same authors suggest that malpositioning is indeed a common occurrence in everyday practice and is more likely with increasing patient body mass index (BMI) [80, 81]. Increased BMI is independently linked to increased rates of post-operative arthroplasty complications, compounded by errors in component placement resulting in suboptimal positioning [81].
The second critical element is the ability of the set up to
The pelvis is exposed to many discrete deforming forces during a conventional THA which may result in iatrogenic pelvic tilt [3, 84]. The process of mechanical cup reaming and implant impaction are obvious examples [84], but ‘strong’ traction from exposure-permitting retractors are also a recognised culprit [84, 86]. While traction for safe exposure may be an unavoidable evil during primary THA, authors who have considered this important force mechanism recommend releasing or ‘backing off’ retractor tension during the critical stage of definitive cup impaction [85], which may permit some measure of tilt correction [84]. The 2019 work of Della Valle et al. however suggests that retractor removal is unlikely to facilitate complete correction of anterior roll which had been induced earlier during the case [82].
Thirdly, the surgeon must be able to accurately, consistently and reproducibly introduce the acetabular component with the correct intended 3D orientation and then impact it whilst precisely maintaining this. As a fundamental tenant of the assumption that surgeons can reliably perform this task Somerville et al. [86] explored the accuracy with which a cohort of experienced trauma and arthroplasty surgeons visually assessed cup anteversion and inclination insertion angles [86]. There was great variability amongst the group with results ranging from ‘very poor’ to ‘very good’ with only moderate inter-observer reproducibility [86]. There have been many proposed methods for improving the precision and/or reproducibility of cup insertion. Such measures have included: following anatomical landmarks [83], the use of intra-operative imaging [79], manual instrumentation jigs and alignment guides [67] or the use of computer-assisted navigation [87]. The most commonly cited anatomic landmark for cup insertion remains the transverse acetabular ligament (TAL) [44]. The value of this local feature has been questioned however, the earlier work of Epstein et al. suggesting the TAL was only appreciably present in 47% of osteoarthritic hips [88] and that, even when it was identified, its presence and recognition did not improve the attainment of target cup position [88]. They concluded that cup orientation using the TAL was no more accurate than an unassisted freehand insertion technique [88], with the subsequent work of Beverland et al. actively recommending against using the TAL to determine final cup inclination [44].
While the use of real-time imaging has been suggested as a potentially useful step to improve the accuracy of final cup position although this too is not without its inherent challenges. Difficulty in the physical process of introducing imaging equipment into sterile fields and capturing meaningful images (i.e. accurately perpendicular to the long axis of the pelvis), concerns regarding radiation exposure and fundamental problems with the interpretation of an image captured in a decubitus position (as compared to the ‘routine’ AP supine or standing states) are all noteworthy considerations. While some authors advocate the use of imaging routinely [89, 90] (most often fluoroscopy [80]) as an intra-operative aid—especially in the setting of a high BMI patient [80]—others have suggested limited utility through such means citing mismatch between apparent ‘during surgery’ and post-operative radiographic cup orientation [68]. Hayakawa et al. suggested mean errors of >5° in both cup inclination and anteversion perception using intra-operative radiographs versus the post-operative gold standard [91] concluding that in-theatre determinations may not reflect post-operative targets.
Using conventional instrumented cup implantation techniques there are many proprietary differences between implant systems which cloud comparability. In essence, the AP inclination angle (i.e. ‘lateral opening’ or ‘abduction’ angle) is visually-appreciated as the angle between the cup insertion handle and the sagittal plane [67] (Figure 3). Critically, if at the time of cup insertion the PSP is not (or is no longer) parallel to the floor, an error of component placement will occur [67]. Body axis alignment (or appreciation thereof) directly influences cup version, as can changes in pelvic flexion and extension.
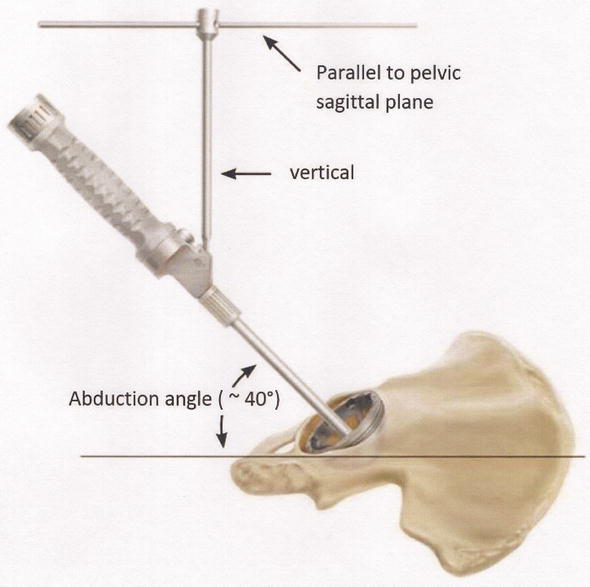
Figure 3.
Cup insertion angles using instrumented alignment towers.
Whilst a relatively recent addition to the arthroplasty surgeon’s armamentarium in many parts of the world, the use of intra-operative computer-assisted hip navigation provides another means for aiding cup insertion [75, 92]. In its two most basic forms, such systems use either pre-operative ‘imaging informed’ or ‘imageless’ [73, 93] approaches. As with accepted total knee arthroplasty (TKA) applications, both portable (i.e. ‘mini-nav’ [94, 95]) and ‘full navigation’ systems are available for use during hip surgery. Whilst large volume data are still pending, early applications of navigated THA suggest consistent improvements in achieving the desired insertion orientation [93, 94, 96] with significantly less (p < 0.001) deviations from target [92, 97]. Most commercially-available navigation systems reference the APP which provides the frame-of-reference orientation for later angular measurement [51, 73] although the paper by Vigdorchik and colleagues suggests that the perpendicular hip-shoulder-axis may actually be the more accurate and consistent registration plane [98]. The well-performed 2020 prospective randomised control trial by Tanino et al. compared the accuracy of a portable, accelerometer-based navigation system with that of conventional instrumented techniques [92]. While adding an average of 10 operative minutes to each case [92], the use of navigation was associated with significant improvements in attainment of target cup position [92]—a sentiment supporting the landmark earlier work of Jolles et al. in 2004 [99]. One of the key benefits of contemporary THA navigation [75, 92] likely lies in the ability of such systems to track pelvic movement during surgery and provide ‘corrective’ measurements [100]. In many cases, the system has the ability to recognise the occurrence and magnitude of pelvic positional changes, even when such movement is below the threshold of unaided surgeon perception. While many authors (and users) feel that intra-operative navigation stands as the best widely available tool for accurate cup implantation [73], such systems do have their own inherent shortcomings including a user learning-curve, system failures, loss of tracker position and poor reliability with increasing pelvic tilt [95, 101]. The integration of biplanar EOS-based imaging methods with existing navigation applications (NAVEOS; VA, USA) is an exciting novel pairing [69] which has been touted to further simplify cup placement with increased 3D accuracy in a lateral decubitus position [69] however, this technology needs further, rigorous, validation before wider adoption can be championed.
6. Discussion
Worldwide, the majority of primary THA is still performed in the lateral position [44] although it has been shown that this position is associated with the greatest degree of unintended intra-operative pelvic movement [74, 78, 92, 102]. Accurate acetabular cup orientation is critical in THA for good clinical results [103, 104] and most authors acknowledge that this can often be a difficult task [47]. Pelvic tilt alters apparent cup position [74] and may subsequently result in suboptimal placement [100]. While the operative approach itself whilst in the decubitus orientation is also an independent consideration for movement (more so with posterior versus anterolateral approaches [76]), failure to recognise changes in pelvic position introduces the potential for erroneous cup placement [76], compounding surgeon insertion errors. Poor acetabular component placement has been linked to a number of post-operative adverse outcomes [49, 94] including accelerated bearing wear [23, 45, 70, 91, 105, 106] and dislocation risk [45, 70, 91, 92, 105, 107, 108] mechanical impingement [108], decreased functional range-of-movement [57, 70, 92, 105], component migration [91], poor joint function [106], and metal ion toxicity [106]. Regardless of the target orientation, the ability to reliably and predictably achieve the desired acetabular component position is crucial to successful THA [80, 83, 109].
As discussed previously herein, final cup position is substantially influenced by the ‘on table’ patient positioning [45, 77], including the initial set up [44, 76, 80]. Despite the best efforts of surgeons/theatre teams, it remains the case that a pelvis will often move unintendedly during the performance of a THA [46, 76]—in spite of seemingly well applied and tensioned positioning devices. Surgeons must remain cognisant to this reality [84]. While several novel devices have been proposed, to date, there exists no mainstream, low-risk accepted method for ensuring a ‘true’ lateral position at the start of each case [68]. Statistically, a pelvis is (far) more likely to roll anteriorly (p < 0.001) during a THA in the decubitus set up [46] and this forward tilt is likely progressive across the operation [82]. It has been demonstrated that the greatest source of error occurs when the PSP is no longer horizontal at time of cup insertion [110]. While it has been proposed that such sequential loss of starting position likely progresses until at least the point of definitive cup and liner insertion, few quantitative data support this at this stage—another inviting opportunity for future research. Pure anterior pelvic roll has been shown to influence cup anteversion to a greater extent than inclination [75]. It is accepted that major pelvic movement may have an effect on the final cup insertion position [85] through surgeon perceptual error. Given the common anterior roll mechanism seen, this consequently leads to an underestimation of cup anteversion [82], with the degree of error directly related to the magnitude of pelvic tilt [49, 78, 107].
How far does an average pelvis move during a routine, primary, THA? Several previous authors have attempted to quantify ‘normal’ ranges of unintended pelvic movement during THA [74, 76, 82, 85] and then to propose acceptable ‘cut offs’ to define clinically-important variation [75]. Anterior (or posterior) pelvic tilt alters the position of the cup in the sagittal plane [111] which has a direct impact on version perception. In case series’ including 67–100 hips [74, 75, 76, 82, 85] previous works have reported median pelvic tilt values during surgery of >4° [46, 82], however mean values and maximum observed tilts ranged broadly between studies—often approaching 20° for the latter [82]. Such studies show 41–57% of cases rolling anteriorly >5° [74, 75, 80], with 21–38% by >10° [46, 75, 83]. Otero’s paper reported 15.4% of cases with 10–20° of tilt and 2.8% with >20° [83]. In interpreting these errors, Grammatopoulos et al. suggested that a > 10° anteversion error had a 3.5 odds ratio of the final cup position falling outside of the target safe zone [46]. Using widely accepted mathematical conversion factors [111, 112], 1° of pelvic tilt results in a 0.7–0.8° change in final anteversion. Given the longstanding surgical goal of achieving target anteversion +/− 10° (see Lewinnek and others [113]), an unappreciated intra-operative pelvic tilt of just 13° would therefore be enough to see an otherwise perfectly centred cup fall outside of the ‘safe’ anteversion range.
Inconsistency in initial patient set up [76] (i.e. with non-perpendicular ‘true’ lateral decubitus positioning) linked with a subsequent change in the pelvic position during the operative process (i.e. movement) likely contributes a substantial burden of the variation seen in final cup position [76] despite otherwise technically sound surgical technique. Uniaxial pelvic tilt has been specifically associated with unintended errors in cup version [112]. A high correlation between direct pelvic tilt and version angle (R2 = 0.995, p < 0.001) [112] has been confirmed and is intrinsically linked to the fact that the negative impact of pelvic tilt can be corrected with relative ease using simple (validated) mathematical algorithms with very high precision [112]. Until recently, the challenge however has remained the ability to
Using standard navigation, it is possible to determine pelvic inclination and tilt by calculating the angular difference between the anatomic frontal plane and true horizontal (i.e. floor) [87]. Modern navigation systems—especially those using accelerometer-based technologies—provide the valuable added benefit of measuring the relative change in the pelvic position independently from data captured from the fixed pelvic tracker(s). Measurement of pelvic tilt during THA allows corrective algorithms to re-calculate the cup insertion angles to correct for the error introduced by pelvic movement and have been shown to improve the accuracy of component placement as per the intended target [42, 111]. The large 2010 study by Zhu and colleagues explored the quantitative value of navigation during THA in a cohort approaching 500 hips [111]. While these authors reported a mean intra-operative tilt of just under 5°, the observed range was from 25° of posterior tilt through to 20° of anterior (i.e. a 45° unintended error range) [111]. Over 25% of patients rolled 6–9°, while over 16% moved more than 10°. It has not yet been definitively established what the perceptual tolerances of visual assessment of pelvic tilt may be by surgeons (or varying levels of experience) although it seems clear that deficiencies in this key skill likely have a negative influence on intended cup implantation position [57].
While much research, attention and interest has centred around pelvic tilt during surgery, the important role of pelvic adduction is rarely assessed or considered [82, 115]. Given that the acetabular cup is a 3D element, inserted with intended orientation goals in 3D, it is conceivable that unintended pelvic movement in any direction may have negative consequence on final cup position [86]. Mathematically, unappreciated pelvic adduction can increase radiographic inclination [114] which may have consequences for final bearing stability [115]. In a routine posterior approach to the hip (in a lateral decubitus position) the relatively wider pelvis as compared to the lower limbs tends to see the uppermost hemipelvis drift into adduction [114]. The previous work of O’Neill and colleagues (2018) assessed the pelvic movement in 270 consecutive primary THAs suggesting that none of their cases showed pelvic abduction with a mean adduction change of 4.4° [115]. This finding was similar to other authors who reported average adduction angles of 2.5–6.7° [82, 84]. It is generally felt that these smaller magnitude changes have a lesser impact on inclination than do comparable movements involving pelvic tilt.
Current research would support the notion that anterior pelvic roll occurs incrementally across the case from set up to definitive implant insertion. The descriptive work of Grammatopoulos et al. suggested a mean angular movement from set up to implant insertion of 9° (sd 6) [76]. Others have suggested similar changes [85]. The later work of Schloemann and colleagues suggested that more than just 5° of change may be ‘clinically significant’ [89] supporting the suggestion that such unaccounted for angular change may facilitate introduction of critical errors in target cup placement [75, 89]. Several authors have recommended that the highest (and most consistent) level of attainment of target cup position may perhaps be achieved using the combination of an assistive anatomical plane (pelvic) positioner and navigation [100, 103]. Iwakiri and colleagues suggested such an approach was reliably simple, consistent, economical and non-invasive [103].
Despite the focus of hip navigation on radiographic outcomes and intra-operative changes, the critical consideration of patient body habitus must be considered. Most authors agree that increasing patient BMI influences the likelihood of unintended pelvic positional change during surgery itself [84] and strongly correlates with subsequent errors in target cup orientation attainment [80, 85]. The extended size of bariatric tissue retractors for surgical exposure [84, 85] (and sometimes the force applied to them) and direct soft tissue impingement can worsen the magnitude of positional movement [116]. However, high BMI alone cannot be blamed for all of the issues noted with unintended pelvic positional change—the 2019 work of Schloemann et al. showed clinically-relevant anterior pelvic roll in a cohort with a mean BMI of just 20 [89]. Similarly, other authors have suggested no clear association between BMI and pelvic movement [75, 82, 117]. Regardless, obesity is just one factor so far linked to pelvic movement during THA surgery—with evidence to show that low volume surgeons and the surgical approach employed are also recognised cofactors [30, 118].
7. Looking to the future …
The future for hip arthroplasty appears exciting, especially as appropriately-employed technologies facilitate further improvements in planning, precision and intra-operative execution. Historical two dimensional (2D) templating and planning has already been shown to be far less accurate than modern 3D equivalents [119, 120, 121]. The evolution to more universal 3D standards is likely to incrementally improve surgical planning [122, 123, 124] as such technologies become more mainstream. The cutting edge integration of artificial intelligence algorithms into the pre-operative decision making pathways may represent further advancement still [124]. Similarly, as real-time computer navigation is taken up more broadly many anticipate improved attainment of target cup placement [87] (in a similar fashion to accuracy improvements that were seen during the evolution of TKA navigation). Despite great enthusiasm in some spheres, navigated arthroplasty is not without its inherent problems and limitations. Tracker pin site placement and loosening [102] continue to undermine case-by-case precision with only small positional changes resulting in magnified degradation in accuracy. As with other bony-mounted navigation applications in other parts of the body, site fractures, wound and pin site issues post-operatively also plague use and present technique-specific challenges [101].
Some supporters of technology have suggested that formal (‘full’) hip navigation may be unnecessary, suggesting that less invasive and less time consuming alternatives are already available to improve operative precision. Using a simple off-the-self smartphone with basic accelerometer capability, Peters et al. in 2012 reported a series of 50 THAs suggesting their novel technique was simple, ‘quick and accurate’, reporting that ‘all’ cases were able to achieve less than 5% deviation from the intended pre-operative plan [47]. Similar work by O’Neill et al. using a simple digital inclinometer reported achieving target cup position within 2.5° in 88% of cases [110] and showed positive statistically-significant differences as compared to conventional instrumented approaches. Contrasting CT-based full navigation with ‘imageless’ accelerometer (mini) navigation however, the recent work by Testsunaga et al. suggested the latter lacked the accuracy of image-based techniques [102] but it was unclear whether the precision-versus-target cup position translated to meaningful clinical benefit. Equally, the potential improvement in accuracy must be weighed against the time, expense and radiation exposure associated with CT-based pre-op imaging. As point-of-care image registration approaches continue to improve with software and algorithm refinement, ease of use and reliable user accuracy will likely improve in parallel.
Regardless of the fundamental imaging method employed (i.e. plain X-ray, augmented X-ray, CT or MRI based), the concept of ‘fusing’ advanced or even 3D pre-operative templating with highly-precise intra-operative navigation means poses an exciting state-of-the-art possibility. Such novel approaches—already in clinical use in some domains—exploit the optimal elements of contemporary planning and surgical case execution. Some authors feel this may represent the best of both considerations [43].
Opponents of navigation frequently cite the ‘is the extra angular precision actually worth it’ argument. The now standard use of larger heads, and with increasingly-common selection of dual mobility bearings [59], has arguably improved the stability and mechanical characteristics in many instances perhaps negating the need for such high levels of cup orientation accuracy. Indeed, in their 2013 paper Eilander and colleagues suggested that hip navigation may be an ‘unnecessary’ technical burden, claiming that 82% of the hips included within their comprehensive study had cups within radiographic safe zones using conventional free hand techniques [105]. So far however, this has not been the sentiment shared by most. Finally, the progression to robot-assisted THA surgery [75]—arguably an evolutionary extension of computer-based navigation—may offer further clinical advantages with early science suggesting value, especially in complex cases [125]. This area too requires further research to ensure the evidence base underpinning wider uptake stays ahead of the enthusiastic hype.
8. Conclusions
This comprehensive review of the current literature highlights the following: 1. current techniques and equipment for patient set up in the lateral decubitus position are deficient and, if used poorly, have the potential to cause patient harm. As a result, sagittal plane movement during THAs (i.e. anterior pelvic roll) is currently an accepted shortcoming. Common patterns of sequential pelvic movement during surgery have not been well determined and represent an opportunity for future investigation; 2. the ability of surgeons/surgical teams to visually appreciate (often large) changes in pelvic position with any degree of quantitative precision—in a patient under exclusion draping—is universally unreliable. This is increasingly so in the setting of obesity/high BMI; 3. failure to appreciate such pelvic movement has a direct and tangible effect upon the ability to insert the definitive acetabular component accurately with the intended target position in mind; 4. such unintended component positioning errors likely have a subsequent negative effect on the mechanical parameters of the THA construct and previous evidence would suggest this may lead to increased risk of wear, instability and possibly dislocation (all key determinants of later revision surgery); 5. while the conventional/historical standard for cup insertion has been ‘per the surgeon’s eye’ or using manual alignment jigs, both fail to reliably and accurately appreciate unintended patient movement during the operation itself. Evidence would suggest that—when used correctly—contemporary navigation systems can improve the precision of implant insertion versus target orientations by narrowing outlier ranges and by calculation of corrective parameters to compensate for computer-appreciated pelvic positional change; 6. while used widely in some international settings, intra-operative hip navigation (image informed or imageless) has not yet achieved widespread adoption and still requires rigorous scientific validation to confirm its utility in more general settings and to further refine optimised indications for use. The role of robot-assisted approaches in this context show promise but require more generalised validation.
References
- 1.
Kurmis AP. Thromboprophylaxis after total hip replacement. Journal of Orthopaedic Surgery (Hong Kong). 2010; 18 (1):92-97 - 2.
Lourens EC, Kurmis AP, Yin LW. Clinical impact of pelvic malrotation on radiograph-based preoperative planning for total hip arthroplasty: A proof-of-concept and prudent prediction of acceptable rotation. Indian Journal of Orthopaedics. 2022; 56 :1053-1060 - 3.
Lourens EC, Kurmis AP, Holder C, de Steiger RN. Early revision rates of total hip arthroplasty using Intellijoint HIP® computer navigation system: A Study from the Australian National Joint Replacement Registry of 1911 procedures. Journal of Arthroplasty. 2022 [In press] - 4.
Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. The Lancet. 2007; 370 (9597):1508-1519 - 5.
Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. Journal of Bone and Joint Surgery. American Volume. 1978; 60 :217-220 - 6.
Lum ZC, Pereira GC, Giordani M, Meehan JP. Top 100 most cited articles in orthopaedic surgery: An update. Journal of Orthopaedics. 2020; 19 :132-137 - 7.
Esposito CI, Gladnick BP, Lee YY, Lyman S, Wright TM, Mayman DJ, et al. Cup position alone does not predict risk of dislocation after hip arthroplasty. The Journal of Arthroplasty. 2015; 30 :109-113 - 8.
Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clinical Orthopaedics and Related Research. 2016; 474 (2):386-391 - 9.
Murphy WS, Yun HH, Hayden B, Kowal JH, Murphy SB. The safe zone range for cup anteversion is narrower than for inclination in THA. Clinical Orthopaedics and Related Research. 2018; 476 (2):325-335 - 10.
Tezuka T, Heckmann N, Bodner R, Dorr LD. Functional safe zone is superior to the Lewinnek safe zone for total hip arthroplasty: Why the Lewinnek safe zone is not always predictive of stability. The Journal of Arthroplasty. 2019; 34 :3-8 - 11.
Dorr LD, Callaghan JJ. Death of the Lewinnek "safe zone". The Journal of Arthroplasty. 2019; 34 (1):1-2 - 12.
Giachino M, Aprato A, Revetria TA, Vezzetti E, Massè A, Ulrich L, et al. Dynamic evaluation of THA components by prosthesis impingement software (PIS). Acta Bio-Medica. 2021; 92 (5):e2021295 - 13.
Kennedy JG, Rogers WB, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. The Journal of Arthroplasty. 1998; 13 (5):530-534 - 14.
Meftah M, Yadav A, Wong AC, Ranawat AS, Ranawat CS. A novel method for accurate and reproducible functional cup positioning in total hip arthroplasty. The Journal of Arthroplasty. 2013; 28 (7):1200-1205 - 15.
Ng VY, McShane MA. Understanding acetabular cup orientation: The importance of convention and defining the safe zone. Hip International. 2011; 21 (6):646-652 - 16.
Merle C, Grammatopoulos G, Waldstein W, Pegg E, Pandit H, Aldinger PR, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. Journal of Bone and Joint Surgery. American Volume. 2013; 95 (22):e172 - 17.
McBride A, Flynn J, Miller G, Barnes M, Mackie S. Body mass index and acetabular component position in total hip arthroplasty. ANZ Journal of Surgery. 2013; 83 (3):171-174 - 18.
Grammatopoulos G, Thomas G, Pandit H, Beard D, Gill H, Murray D. The effect of orientation of the acetabular component on outcome following total hip arthroplasty with small diameter hard-on-soft bearings. Bone & Joint Journal. 2015; 97-B (2):164-172 - 19.
Bhaskar D, Rajpura A, Board T. Current concepts in acetabular positioning in total hip arthroplasty. Indian Journal of Orthopaedics. 2017; 51 (4):386-396 - 20.
Scheerlinck T. Cup positioning in total hip arthroplasty. Acta Orthopaedica Belgica. 2014; 80 (3):336-347 - 21.
Jolles BM, Zangger P, Leyvraz PF. Factors predisposing to dislocation after primary total hip arthroplasty: A multivariate analysis. The Journal of Arthroplasty. 2002; 17 :282-288 - 22.
Miki H, Kyo T, Kuroda Y, Nakahara I. Sugano 367 N: Risk of edge-loading and prosthesis impingement due to posterior pelvic tilting after total hip arthroplasty. Clinical biomechanics. 2014; 29 :607-613 - 23.
Kilb BKJ, Kurmis AP, Parry M, Sherwood K, Keown P, Masri BA, et al. Frank Stinchfield award: Identification of the ‘at risk’ genotype for development of pseudotumours around metal-on-metal total hip arthroplasties. Clinical Orthopaedics and Related Research. 2018; 476 (2):230-241 - 24.
Upfill-Brown A, Hsiue PP, Sekimura T, Patel JN, Adamson M, Stavrakis AI. Instability is the most common indication for revision hip arthroplasty in the United States: National trends from 2012 to 2018. Arthroplasty Today. 2021; 11 :88-101 - 25.
Bozic K, Kurtz S, Lau E, Ong K, Vail T, Berry D. The epidemiology of revision total hip arthroplasty in the United States. Journal of Bone and Joint Surgery. American Volume. 2009; 91 (1):128-133 - 26.
Barrack RL, Krempec JA, Clohisy JC, McDonald DJ, Ricci WM, Ruh EL, et al. Accuracy of acetabular component position in hip arthroplasty. Journal of Bone and Joint Surgery. American Volume. 2013; 95 (19):1760-1768 - 27.
Domb BG, El Bitar YF, Sadik AY, Stake CE, Botser IB. Comparison of robotic-assisted and conventional acetabular cup placement in THA: A matched-pair controlled study. Clinical Orthopaedics and Related Research. 2014; 472 (1):329-336 - 28.
Korduba LA, Essner A, Pivec R, Lancin P, Mont MA, Wang A, et al. Effect of acetabular cup abduction angle on wear of ultrahigh-molecular-weight polyethylene in hip simulator testing. The American Journal of Orthopedics. 2014; 43 (10):466-471 - 29.
Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. Journal of the American Academy of Orthopaedic Surgeons. 2003; 11 (2):89-99 - 30.
Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, et al. The John Charnley award: Risk factors for cup malpositioning: Quality improvement through a joint registry at a tertiary hospital. Clinical Orthopaedics and Related Research. 2011; 469 (2):319-329 - 31.
Agarwal S, Eckhard L, Walter W, Peng A, Hatton A, Donnelly B, et al. The use of computer navigation in total hip arthroplasty is associated with a reduced rate of revision for dislocation. Journal of Bone and Joint Surgery. American Volume. 2021; 103 (20):1900-1905 - 32.
Bohl D, Nolte M, Ong K, Lau E, Calkins T, Della VC. Computer-assisted navigation is associated with reductions in the rates of dislocation and acetabular component revision following primary total hip arthroplasty. Journal of Bone and Joint Surgery. American Volume. 2019; 101 (3):250-256 - 33.
Snijders T, van Gaalen S, de Gast A. Precision and accuracy of imageless navigation versus freehand implantation of total hip arthroplasty: A systematic review and meta-analysis. The International Journal of Medical Robotics and Computer Assisted Surgery. 2017; 13 (4):e1843 - 34.
Kurmis AP. Commentary & perspective: Understanding the role of computer-navigation in primary total knee arthroplasty. Journal of Bone and Joint Surgery. American Volume. 2019; 101 :e111(1-2) - 35.
Kurmis AP. Commentary & perspective: Considering the value of imageless, accelerometer-based, intra-operative ‘mini-’ navigation systems in contemporary primary TKA. Journal of Bone and Joint Surgery. American Volume. 2020; 102 (22):e129 - 36.
de Steiger R, Liu Y-L, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. Journal of Bone and Joint Surgery. American Volume. 2015; 97 (8):635-642 - 37.
Ghostine B, Sauret C, Assi A, Bakouny Z, Khalil N, Skalli W, et al. Influence of patient axial malpositioning on the trueness and precision of pelvic parameters obtained from 3D reconstructions based on biplanar radiographs. European Radiology. 2017; 27 (3):1295-1302 - 38.
Nobuhiko S, Takao M, Sakai T. Does CT-based navigation improve the long-term survival in ceramic-on-ceramic THA? Clinical Orthopaedics and Related Research. 2012; 470 (11):3054-3059 - 39.
Iwanna D, Nakamura N, Miki H. Accuracy of angle and position of the cup using computed tomography-based navigation systems in total hip arthroplasty. Computer Aided Surgery. 2013; 18 :187-194 - 40.
Yamada K, Endo H, Tetsunaga T. Accuracy of cup positioning with the computed tomography-based two-dimensional to three-dimensional matched navigation system: A prospective, randomized controlled study. The Journal of Arthroplasty. 2018; 33 :136-143 - 41.
Nakahara I, Kyo T, Kuroda Y. Effect of improved navigation performance on the accuracy of implant placement in total hip arthroplasty with a CT-based navigation system. Journal of Artificial Organs. 2018; 21 (3):340-347 - 42.
Paprosky WG, Muir JM. Intellijoint HIP: A 3D mini-optical navigation tool for improving intraoperative accuracy during total HIP arthroplasty. Medical Devices: Evidence and Research. 2016; 9 :401-408 - 43.
Kurmis AP. Anterior pelvic roll during primary total hip arthroplasty in the lateral decubitus position: A systematic review. Journal of Orthopaedic Surgery. 2022 [In press] - 44.
Beverland DE, O'Neill CKJ, Rutherford M, Molloy D, Hill JC. Placement of the acetabular component. Bone & Joint Journal. 2016; 98-B (1 Suppl. A):37-43 - 45.
Daines BK, Dennis DA. The importance of acetabular component position in total hip arthroplasty. The Orthopedic Clinics of North America. 2012; 43 (5):e23-e34 - 46.
Grammatopoulos G, Gofton W, Cochran M, Dobransky J, Carli A, Abdelbary H, et al. Pelvic positioning in the supine position leads to more consistent orientation of the acetabular component after total hip arthroplasty. Bone & Joint Journal. 2018; 100-B (10):1280-1288 - 47.
Peters FM, Greeff R, Goldstein N, Frey CT. Improving acetabular cup orientation in total hip arthroplasty by using smartphone technology. The Journal of Arthroplasty. 2012; 27 (7):1324-1330 - 48.
Attenello JD, Harpstrite JK. Implications of spinopelvic mobility on total hip arthroplasty: Review of current literature. Hawaiʻi Journal of Health & Social Welfare. 2019; 78 (11 Suppl 2):31-40 - 49.
Shon WY, Sharma V, Keon OJ, Moon JG, Suh DH. Can pelvic tilting be ignored in total hip arthroplasty? International Journal of Surgery Case Reports. 2014; 5 (9):633-636 - 50.
Pierrepont J, Hawdon G, Miles BP, O'Connor B, Baré J, Walter LR, et al. Variation in functional pelvic tilt in patients undergoing total hip arthroplasty. Bone & Joint Journal. 2017; 99-B (2):184-191 - 51.
Blondel B, Parratte S, Tropiano P, Pauly V, Aubaniac JM, Argenson JN. Pelvic tilt measurement before and after total hip arthroplasty. Orthopaedics & Traumatology, Surgery & Research. 2009; 95 (8):568-572 - 52.
Mellano CR, Spitzer AI. How does pelvic rotation or tilt affect radiographic measurement of acetabular component inclination angle during THA? Journal of Orthopaedics. 2015; 12 (4):222-227 - 53.
Kyo T, Nakahara I, Miki H. Factors predicting change in pelvic posterior tilt after THA. Orthopedics. 2013; 36 (6):e753-e759 - 54.
Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthopaedica. 2005; 76 (4):517-523 - 55.
Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. Journal of Bone and Joint Surgery. American Volume. 2008; 90 (2):357-365 - 56.
Maratt JD, Esposito CI, McLawhorn AS, Jerabek SA, Padgett DE, Mayman DJ. Pelvic tilt in patients undergoing total hip arthroplasty: When does it matter? The Journal of Arthroplasty. 2015; 30 (3):387-391 - 57.
Langston J, Pierrepont J, Gu Y, Shimmin A. Risk factors for increased sagittal pelvic motion causing unfavourable orientation of the acetabular component in patients undergoing total hip arthroplasty. Bone & Joint Journal. 2018; 100-B (7):845-852 - 58.
Heckmann N, McKnight B, Stefl M, Trasolini NA, Ike H, Dorr LD. Late dislocation following total hip arthroplasty: Spinopelvic imbalance as a causative factor. Journal of Bone and Joint Surgery. American Volume. 2018; 100 (21):1845-1853 - 59.
Kurmis AP. CORR insights: Is isolated mobile component exchange an option in the management of intraprosthetic dislocation of a dual mobility cup? Clinical Orthopaedics and Related Research. 2020; 478 :288-289 - 60.
Stefl M, Lundergan W, Heckmann N, McKnight B, Ike H, Murgai R, et al. Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone & Joint Journal. 2017; 99-B (1 Supple A):37-45 - 61.
DelSole EM, Vigdorchik JM, Schwarzkopf R, Errico TJ, Buckland AJ. Total hip arthroplasty in the spinal deformity population: Does degree of sagittal deformity affect rates of safe zone placement, instability, or revision? The Journal of Arthroplasty. 2017; 32 :1910-1917 - 62.
Ike H, Dorr LD, Trasolini N, Stefl M, McKnight B, Heckmann N. Spine-pelvis-hip relationship in the functioning of a total hip replacement. Journal of Bone and Joint Surgery. American Volume. 2018; 100 :1606-1615 - 63.
Kleeman-Forsthuber LT, Elkins JM, Miner TM, Yang CC, Jennings JM, Dennis DA. Reliability of spinopelvic measurements that may influence the cup position in total hip arthroplasty. The Journal of Arthroplasty. 2020; 35 (12):3758-3764 - 64.
Lazennec JY, Rousseau MA, Rangel A, Gorin M, Belicourt C, Brusson A, et al. Pelvis and total hip arthroplasty acetabular component orientations in sitting and standing positions: Measurements reproductibility with EOS imaging system versus conventional radiographies. Orthopaedics & Traumatology, Surgery & Research. 2011; 97 (4):373-380 - 65.
Illés T, Somoskeöy S. The EOS™ imaging system and its uses in daily orthopaedic practice. International Orthopaedics. 2012; 36 (7):1325-1331 - 66.
Innmann MM, McGoldrick NP, Ratra A, Merle C, Grammatopoulos G. The accuracy in determining pelvic tilt from anteroposterior pelvic radiographs in patients awaiting hip arthroplasty. Journal of Orthopaedic Research. 2021; 40 (4):854-861 - 67.
McMahon SE, Magill P, Bopf DP, Beverland DE. A device to make the pelvic sagittal plane horizontal and reduce error in cup inclination during total hip arthroplasty: A validation study. Hip International. 2018; 28 (5):473-477 - 68.
Rutherford M, O'Connor JD, Gill HS, Hill J, Beverland D, Lennon AB, et al. Operative and radiographic acetabular component orientation in total hip replacement: Influence of pelvic orientation and surgical positioning technique. Medical Engineering & Physics. 2019; 64 :7-14 - 69.
Billaud A, Verdier N, de Bartolo R, Lavoinne N, Chauveaux D, Fabre T. Acetabular component navigation in lateral decubitus based on EOS imaging: A preliminary study of 13 cases. Orthopaedics & Traumatology, Surgery & Research. 2015; 101 (3):271-275 - 70.
Carcangiu A, D'Arrigo C, Topa D, Alonzo R, Speranza A, De Sanctis S, et al. Reliability of cup position in navigated THA in the lateral decubitus position using the 'flip technique'. Hip International. 2011; 21 (6):700-705 - 71.
Rousseau M-A, Lazennec JY, Boyer P, Mora N, Gorin M, Catonné Y. Optimization of total hip arthroplasty implantation: Is the anterior pelvic plane concept valid? The Journal of Arthroplasty. 2009; 24 (1):22-26 - 72.
Dandachli W, Richards R, Sauret V, Cobb JP. The transverse pelvic plane: A new and practical reference frame for hip arthroplasty. Computer Aided Surgery. 2006; 11 (6):322-326 - 73.
Beckmann J, Lüring C, Tingart M, Anders S, Grifka J, Köck FX. Cup positioning in THA: Current status and pitfalls. A systematic evaluation of the literature. Archives of Orthopaedic and Trauma Surgery. 2009; 129 (7):863-872 - 74.
Kanazawa M, Nakashima Y, Ohishi M, Hamai S, Motomura G, Yamamoto T, et al. Pelvic tilt and movement during total hip arthroplasty in the lateral decubitus position. Modern Rheumatology. 2016; 26 (3):435-440 - 75.
Milone MT, Schwarzkopf R, Meere PA, Carroll KM, Jerabek SA, Vigdorchik J. Rigid patient positioning is unreliable in total hip arthroplasty. The Journal of Arthroplasty. 2017; 32 (6):1890-1893 - 76.
Grammatopoulos G, Pandit HG, da Assunção R, Taylor A, McLardy-Smith P, De Smet KA, et al. Pelvic position and movement during hip replacement. Bone & Joint Journal. 2014; 96-B (7):876-883 - 77.
Rutherford M, O'Connor JD, Hill JC, Beverland DE, Lennon AB, Dunne NJ. Patient positioning and cup orientation during total hip arthroplasty: Assessment of current UK practice. Hip International. 2019; 29 (1):89-95 - 78.
Iwakiri K, Kobayashi A, Ohta Y, Takaoka K. Efficacy of the anatomical-pelvic-plane positioner in total hip arthroplasty in the lateral decubitus position. The Journal of Arthroplasty. 2017; 32 (5):1520-1524 - 79.
Nishikubo Y, Fujioka M, Ueshima K, Saito M, Kubo T. Preoperative fluoroscopic imaging reduces variability of acetabular component positioning. The Journal of Arthroplasty. 2011; 26 (7):1088-1094 - 80.
Lambers P, Jennings R, Bucknill AT. A novel fluoroscopic approach to assessing patient positioning in total hip arthroplasty: Accuracy and the influence of body mass index. Hip International. 2016; 26 (6):550-553 - 81.
Kurmis AP. CORR insights: Statistical methods dictate the estimated impact of body mass index on major and minor complications after total joint arthroplasty. Clinical Orthopaedics and Related Research. 2018; 476 :2430-2431 - 82.
Della Valle AG, Shanaghan K, Benson JR, Carroll K, Cross M, McLawhorn A, et al. Pelvic pitch and roll during total hip arthroplasty performed through a posterolateral approach. A potential source of error in free-hand cup positioning. International Orthopaedics. 2019; 43 (8):1823-1829 - 83.
Otero JE, Fehring KA, Martin JR, Odum SM, Fehring TK. Variability of pelvic orientation in the lateral decubitus position: Are external alignment guides trustworthy? The Journal of Arthroplasty. 2018; 33 (11):3496-3501 - 84.
Brodt S, Nowack D, Jacob B, Krakow L, Windisch C, Matziolis G. Patient obesity influences pelvic lift during cup insertion in total hip arthroplasty through a lateral transgluteal approach in supine position. The Journal of Arthroplasty. 2017; 32 (9):2762-2767 - 85.
Ueno T, Kabata T, Kajino Y, Inoue D, Ohmori T, Yoshitani J, et al. Risk factors for pressure ulcers from the use of a pelvic positioner in hip surgery: A retrospective observational cohort study in 229 patients. Patient Safety in Surgery. 2020; 14 :10 - 86.
Somerville CM, Geddes JA, Tofighi M, Boddu K. Accuracy and reproducibility of visual estimation of the acetabular cup positioning in total hip arthroplasty on plain radiographs by orthopaedic surgeons. Journal of Perioperative Practice. August 16, 2021:17504589211026074. DOI: 10.1177/17504589211026074 - 87.
Chen E, Goertz W, Lill CA. Implant position calculation for acetabular cup placement considering pelvic lateral tilt and inclination. Computer Aided Surgery. 2006; 11 (6):309-316 - 88.
Epstein NJ, Woolson ST, Giori NJ. Acetabular component positioning using the transverse acetabular ligament: Can you find it and does it help? Clinical Orthopaedics and Related Research. 2011; 469 (2):412-416 - 89.
Schloemann DT, Edelstein AI, Barrack RL. Changes in acetabular orientation during total hip arthroplasty. Bone & Joint Journal. 2019; 101-B (6_Supple_B):45-50 - 90.
Seo H, Naito M, Nakamura Y, Kinoshita K, Nomura T, Minokawa S, et al. New cross-table lateral radiography method for measuring acetabular component anteversion in total hip arthroplasty: A prospective study of 93 primary THA. Hip International. 2017; 27 (3):293-298 - 91.
Hayakawa K, Minoda Y, Aihara M, Sakawa A, Ohzono K, Tada K. Acetabular component orientation in intra- and postoperative positions in total hip arthroplasty. Archives of Orthopaedic and Trauma Surgery. 2009; 129 (9):1151-1156 - 92.
Tanino H, Nishida Y, Mitsutake R, Ito H. Portable accelerometer-based navigation system for cup placement of total hip arthroplasty: A prospective, randomized, controlled study. Journal of Arthroplasty. 2020; 35 (1):172-177 - 93.
Parvizi J, Benson J, Muir J. A new mini-navigation tool allows accurate component placement during anterior total hip arthroplasty. Medical Devices: Evidence and Research. 2018; 11 :95-104 - 94.
Tanino H, Nishida Y, Mitsutake R, Ito H. Accuracy of a portable accelerometer-based navigation system for cup placement and intraoperative leg length measurement in total hip arthroplasty: A cross-sectional study. BMC Musculoskeletal Disorders. 2021; 22 (1):299 - 95.
Asai H, Takegami Y, Seki T, Ishiguro N. Pelvic tilt reduces the accuracy of acetabular component placement when using a portable navigation system: An in vitro study. Arthroplasty Today. 2021; 7 :177-181 - 96.
Cross MB, Schwarzkopf R, Miller TT, Bogner EA, Muir JM, Vigdorchik JM. Improving registration accuracy during total hip arthroplasty: A cadaver study of a new, 3-D mini-optical navigation system. Hip International. 2018; 28 (1):33-39 - 97.
Mei XY, Etemad-Rezaie A, Safir OA, Gross AE, Kuzyk PR. Intraoperative measurement of acetabular component position using imageless navigation during revision total hip arthroplasty. Canadian Journal of Surgery. 2020; 64 (4):442-448 - 98.
Vigdorchik JM, Sculco PK, Inglis AE, Schwarzkopf R, Muir JM. Evaluating alternate registration planes for imageless, computer-assisted navigation during total hip arthroplasty. The Journal of Arthroplasty. 2021; 36 (10):3527-3533 - 99.
Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clinical Orthopaedics and Related Research. 2004; 426 :174-179 - 100.
Tsukamoto M, Kawasaki M, Suzuki H, Fujitani T, Sakai A. Proposal of accurate cup placement procedure during total hip arthroplasty based on pelvic tilt discrepancies in the lateral position. Scientific Reports. 2021; 11 (1):13870 - 101.
Kurmis AP. Retained pelvic pin site debris after navigated THA: Masquerading as an early-stage chondrosarcomatous lesion. Journal of Postgraduate Medicine. 2020; 66 (4):215-217 - 102.
Tetsunaga T, Yamada K, Tetsunaga T, Furumatsu T, Sanki T, Kawamura Y, et al. Comparison of the accuracy of CT- and accelerometer-based navigation systems for cup orientation in total hip arthroplasty. Hip International. 2021; 31 (5):603-608 - 103.
Iwakiri K, Kobayashi A, Ohta Y, Minoda Y, Takaoka K, Nakamura H. Efficacy of a pelvic lateral positioner with a mechanical cup navigator based on the anatomical pelvic plane in total hip arthroplasty. The Journal of Arthroplasty. 2017; 32 (12):3659-3664 - 104.
Sheridan GA, Hanlon M, Welch-Phillips A, Spratt K, Hagan R, O’Byrne JM, et al. Identification of protective and ‘at risk’ HLA genotypes for the development of pseudotumours around hip resurfacings—A case-control study. Journal of Arthroplasty. 2022 [In press] - 105.
Eilander W, Harris SJ, Henkus HE, Cobb JP, Hogervorst T. Functional acetabular component position with supine total hip replacement. Bone & Joint Journal. 2013; 95-B (10):1326-1331 - 106.
Tiberi JV, Pulos N, Kertzner M, Schmalzried TP. A more reliable method to assess acetabular component position. Clinical Orthopaedics and Related Research. 2012; 470 (2):471-476 - 107.
Yang G, Li Y, Zhang H. The influence of pelvic tilt on the anteversion angle of the acetabular prosthesis. Orthopaedic Surgery. 2019; 11 (5):762-769 - 108.
Foissey C, Batailler C, Fary C, Luceri F, Servien E, Lustig S. Transitioning the total hip arthroplasty technique from posterior approach in lateral position to direct anterior approach in supine position-risk factors for acetabular malpositioning and the learning curve. International Orthopaedics. 2020; 44 (9):1669-1676 - 109.
Goyal P, Lau A, Naudie DD, Teeter MG, Lanting BA, Howard JL. Effect of acetabular component positioning on functional outcomes in primary total hip arthroplasty. The Journal of Arthroplasty. 2017; 32 (3):843-848 - 110.
O'Neill CKJ, Hill JC, Patterson CC, Molloy DO, Gill HS, Beverland DE. Reducing variability in apparent operative inclination during total hip arthroplasty: Findings of a randomised controlled trial. Hip International. 2018; 28 (3):234-239 - 111.
Zhu J, Wan Z, Dorr LD. Quantification of pelvic tilt in total hip arthroplasty. Clinical Orthopaedics and Related Research. 2010; 468 (2):571-575 - 112.
Xu J, Su B, Zhang W, Sun H, Li D, Cai Z, et al. 3D simulation of radiographic projections to test and reduce the effect of pelvic tilt on the accuracy of cross-table lateral radiography. BMC Musculoskeletal Disorders. 2020; 21 (1):843 - 113.
Sharma AK, Cizmic Z, Dennis DA, Kreuzer SW, Miranda MA, Vigdorchik JM. Low dislocation rates with the use of patient specific "safe zones" in total hip arthroplasty. Journal of Orthopaedics. 2021; 27 :41-48 - 114.
Hill JC, Gibson DP, Pagoti R, Beverland DE. Photographic measurement of the inclination of the acetabular component in total hip replacement using the posterior approach. Journal of Bone and Joint Surgery. British Volume (London). 2010; 92 (9):1209-1214 - 115.
O'Neill CKJ, Magill P, Hill JC, Patterson CC, Molloy DO, Gill HS, et al. Correction of pelvic adduction during total hip arthroplasty reduces variability in radiographic inclination: Findings of a randomised controlled trial. Hip International. 2018; 28 (3):240-245 - 116.
Woerner M, Weber M, Sendtner E, Springorum R, Worlicek M, Craiovan B, et al. Soft tissue restricts impingement-free mobility in total hip arthroplasty. International Orthopaedics. 2017; 41 (2):277-282 - 117.
Kishimura Y, Minoda Y, Mizokawa S, Sugama R, Ohta Y, Nakamura H. Cup alignment in total hip arthroplasty using the muscle-sparing modified Watson-Jones approach-comparison between lateral and supine positions. International Orthopaedics. 2019; 43 (11):2477-2483 - 118.
Davda K, Smyth N, Cobb JP, Hart AJ. 2D measurements of cup orientation are less reliable than 3D measurements. Acta Orthopaedica. 2015; 86 (4):485-490 - 119.
Malik A, Jain N, Scharschmidt TJ, Li M, Glassman AH, Khan SN. Does surgeon volume affect outcomes following primary total hip arthroplasty? A systematic review. Journal of Arthroplasty. 2018; 33 :3329-3342 - 120.
Kurmis AP. Orthopaedic utilisation of three-dimensional image displays reconstructed from magnetic resonance imaging. The Radiographer. 2002; 49 (2):67-71 - 121.
Kurmis AP. The developing role of knee MRI in musculo-skeletal radiology: The progression to 3-D imaging. The Radiographer. 2001; 48 (1):21-28 - 122.
Kurmis AP, Slavotinek JP. Reconstructed three-dimensional MR images: Application to simulated tibial plateau depression fractures. Radiography. 2004; 10 (2):95-101 - 123.
Kurmis AP, Hearn TC, Grimmer K, Reynolds KJ. Dimensional measurement of structural features of the ovine knee using three-dimensional reconstructed imaging: Intra- and inter-observer repeatability. Radiography. 2004; 10 (4):269-276 - 124.
Kurmis AP, Ianunzio JR. Artificial intelligence in orthopaedic surgery: Evolution, current state and future directions. Art. 2022; 4 (1):9 - 125.
Zhou Y, Shao H, Huang Y, Deng W, Yang D, Bian T. Does robotic assisted technology improve the accuracy of acetabular component positioning in patients with DDH? Journal of Orthopaedic Surgery (Hong Kong). 2021; 29 (2):23094990211025325