The intraoperative conditions which result in stiff TKA.
Abstract
Total knee arthroplasty (TKA) is one of the most successful surgical procedures with effective treatment in patients suffering from end-stage knee osteoarthritis. The goal of the operation is to improve pain, correct the deformity, and increase function. However, complications after surgery are the important factors related to dissatisfied TKA. Stiffness, periprosthetic joint infection (PJI), and periprosthetic fracture are among the most common complications following TKA and usually raise issues as concern points for both patients and the surgeons. Each complication needs precise assessment and specific care to prevent further serious issues. In this chapter, the authors will focus and describe all of these three frequent complications in details from their definition to management.
Keywords
- total knee arthroplasty
- complications
- stiffness
- periprosthetic joint infection
- periprosthetic fracture
1. Introduction
There is approximately 20 percent of the patients with dissatisfaction following TKA whether pain or having postoperative problems [1, 2]. In the previous literatures, reporting of complications and adverse events after TKA was not standardized with several definitions being proposed. Healy et al. published a list of 22 TKA complications and their standardized definitions including these three common problems, which were endorsed by The Knee Society to improve quality measurement and consistent with ICD-9 codes [3]. Stiff TKA could produce pain and diminish functional ability, whereas PJI and periprosthetic fracture might cause severe morbidity. Early detection and appropriate management are the key success to resolve these problems which would enhance patient’s outcome and improve satisfaction.
2. Stiffness
Normal knee range of motion (ROM) ranges from 0 to 140 degrees, while achievement of postoperative ROM from 0 to 110 degrees can be defined as success TKA [4]. In general, a minimum of 90 degrees of knee flexion is required for functional recovery in daily activities, as 83 degrees of knee flexion is required for going up and down stairs and 93 degrees for sitting, which were demonstrated by a biomechanical study [5, 6]. Stiffness after TKA has variable incidence, ranging from 1.3 to 5.3 percent, but some literature proposed up to 60 percent of patients who suffered from stiff TKA [7]. These variables may cause by a variety of definitions as there was absolutely no consensus on degrees of knee flexion limitation defined as stiff TKA. The standardized definition by the TKA Complications Workgroup of The Knee Society described that limitation of ROM as reported by the patient with physical examination showed extension restriction to 15 degrees short of full extension or flexion less than 90 degrees were defined as stiffness. However, this definition could not be applicable if the preoperative arc of motion is less than 75 degrees [3].
2.1 Factors
The factors or etiologies related to stiff TKA could be categorized into three phases: preoperative, intraoperative, and postoperative periods [8]. Each period also has its specified causes and different management. Surgeons should evaluate the patients carefully for the proper treatment.
2.1.1 Preoperative period
Preoperative ROM limitation is the most important risk factor for postoperative stiffness [4, 9]. Patients with a greater degree of preoperative ROM had superior postoperative ROM and functional scores, with less complications. Only 71.4 percent of patients with preoperative ROM less than 90 degrees would achieve postoperative ROM at least 90 degrees, while more than 90 percent of patients with preoperative ROM greater than 90 degrees could perform ROM more than 90 degrees postoperatively [10]. Lee et al. demonstrated that 33 percent of patients with preoperative ROM less than 50 degrees developed either superficial or deep infection, as well as skin necrosis after the operation, whereas only 13 percent of patients with preoperative ROM between 50 and 90 degrees suffered from these complications [11]. The cause of stiffness before surgery is also one of the considerable factors for postoperative stiffness. The same study by Lee et al. showed patients with osteoarthritis or rheumatoid arthritis had greater postoperative ROM than patients with prior infectious arthritis or traumatic arthritis significantly [11]. Patients with younger age, absence of diabetes mellitus, and lower preoperative walking limitations were found to be the additional predictors with better postoperative ROM [12]. Moreover, obesity might be another factor influencing postoperative ROM. Järvenpää et al. proposed patients with body mass index (BMI) greater than 30 kg/m2 had poorer postoperative ROM at 1-year follow-up approximately 6 degrees than patients with less BMI [13].
2.1.2 Intraoperative period
At the time of surgery, technical errors during the bone cut, soft tissue procedure, and implantation, which relate to an imbalance in flexion and extension gaps, are the most frequent causes of postoperative stiffness. All of these conditions may result in limitation of motion both flexion and extension after TKA (Table 1) [4, 9].
| Bone cut | Soft tissue procedure | Implantation |
|---|---|---|
|
|
|
Table 1.
2.1.3 Postoperative period
There are several factors causing stiffness following TKA in this period, including inadequate rehabilitation and poor patient motivation, deep infection, arthrofibrosis, complex regional pain syndrome (CRPS), associated stiffness or pain derived from the adjacent joints or spine that alters knee motion, and heterotropic ossification (HO) [14]. Adequate postoperative pain management is essential in improving functional recovery and achieving rehabilitation protocol, especially knee motion enhancement [15]. Deep infection or PJI is one of the conditions leading to difficulty in ROM with chronic dull pain. It should be considered, especially in patients who developed stiffness after achieving adequate ROM [4, 14]. The details of this condition are described later in this chapter.
Arthrofibrosis after TKA is the most common cause of stiffness with an incidence ranging from 1.2 to 17 percent [9]. The etiology is multifactorial and the exact pathophysiology is unclear. Patients with poor preoperative ROM, higher complexity surgery, and a history of previous knee surgery increase the risk of excessive fibrous tissue formation after TKA. The theory of developing arthrofibrosis is disruption of cytokines and growth factors signaling cell growth, differentiation, and death, resulting in uncontrolled proliferation of fibroconnective tissue [16]. The histology is characterized by metaplasia of calcified tissue, myofibroblasts, and excessive fibrosis, with the increasing number of macrophages and lymphocytes in the periarticular tissue [17, 18]. The clinical manifestation is broad spectrum, from a localized lesion to a generalized involvement of the entire joint, and results in the formation of extensive extra-articular fibrous tissue.
Recently, there is no gold standard for diagnosis of arthrofibrosis, and also no effective method to prevent the idiopathic arthrofibrosis after TKA, apart from patient education and early mobilization [4].
2.2 Treatment
Initial evaluation of stiff TKA to assess the causes is necessary before management. A correct diagnosis leads to correct treatment. The evaluation should review back to the preoperative status of the patient, especially the risk factors mentioned above. The radiological examination should perform in case of suspicious mechanical problems from surgical errors of bone cut and implantation. Do not hesitate to work up for PJI if infection or wound-related complications that predispose the patient to infection are suspected [4, 9].
There are various treatment options for stiff TKA: manipulation under anesthesia (MUA), arthroscopic arthrolysis, open arthrolysis, and revision surgery [4].
2.2.1 Manipulation under anesthesia (MUA)
The purpose of MUA is to break immature adhesions within the knee in patients who disadvantage of self-training or regular rehabilitation programs and accelerate the initial rehabilitation process [19]. This procedure should be performed within 6–8 weeks after initial TKA before the development of mature adhesions which increases the likelihood of complications after MUA, especially periprosthetic fractures or rupture of the extensor mechanism [20]. Aggressive rehabilitation is necessary to prevent further and recurrent stiffness. A systematic review by Fitzsimmons et al. showed a mean gain in knee motion from 30 to 47 degrees after MUA [7].
2.2.2 Arthroscopic arthrolysis
Arthroscopic arthrolysis is a minimal invasive surgery that resects fibrosis directly in the suprapatellar pouch, medial and lateral gutters, and also in the intercondylar groove [4]. The indication of this operative procedure is painless, stiff TKA after non-progression of conservative treatment for 3 months. Disadvantage is inadequate arthrolysis because of poor access to the posterior structure and the area above the suprapatellar pouch [9]. A systematic review demonstrated improvement of overall ROM between 18.5 and 60 degrees, which also achieved 30.8–42 degrees even performing arthroscopic arthrolysis after 1 year of index TKA [7].
2.2.3 Open arthrolysis
Open lysis of adhesions is recommended in case of severe ROM limitation which impedes the use of arthroscope without component malposition and after the failure of conservative treatment. This operative procedure can provide a broad assessment of the knee joint and fibrosis resection should be performed meticulously. However, exposure to the joint may be difficult from adhesions and need further operative technique, for example, tibial tubercle osteotomy, quadriceps snip, or VY-plasty [4, 9]. A systematic review by Fitzsimmons et al. showed an average increasing of ROM between 19 and 31 degrees after open arthrolysis [7].
2.2.4 Revision surgery
This is the final treatment option reserved for stiffness from surgical errors that need to be corrected. Accurate analysis of the errors is required for planning the revision correctly to meet the patient’s satisfaction [4].
3. Periprosthetic joint infection (PJI)
PJI is a serious complication and is considered one of the most common causes of revision surgery following the failure of primary TKA [21]. The incidence of PJI after primary knee replacement is ranging from 0.85 to 2.2 percent [22], with a higher rate up to 9 percent in revision cases [23]. Despite a small incidence of infection following TKA, the trend of revision due to PJI was rising by 2.5-fold in the past decade [22]. This problem illustrates an increasing and substantial treatment burden to both orthopedic surgeons and the patients, as well as the health service system.
A systematic review and meta-analysis by Kunutsor et al. showed patients with smoking, BMI >30 kg/m2, diabetes, depression, steroid use, previous joint surgery, and frailty were the significant risk factors associated with the long-term developing PJI [24]. A study by Rosteius et al. demonstrated the most common pathogen found in PJI after TKA was methicillin-susceptible
3.1 Diagnosis
Recently, there is no gold standard for the diagnosis of PJI [21]. The Musculoskeletal Infection Society (MSIS) and the Infectious Diseases Society of America (IDSA) have previously developed criteria to standardize the definition of PJI in 2011 and 2013 [26, 27], together with an International Consensus Meeting on PJI in 2013 [28]. The latest consensus in 2018 proposed a new scoring-based definition for PJI after emerging of new diagnostic tests. Two positive cultures of the same organism or the presence of a sinus tract were considered as major criteria and a definite diagnosis of PJI. The minor criteria consisted of laboratory tests either serum or synovial fluid which were weighted differently. An elevated serum C-reactive protein (CRP) or D-dimer received 2 points, whereas an elevation of erythrocyte sedimentation rate (ESR) weighted 1 point. Furthermore, an elevated synovial white blood cell (WBC) count or leukocyte esterase (LE) was considered 3 points. The other diagnostic tests for synovial fluid were a positive alpha-defensin, an elevated synovial polymorphonuclear (PMN) percentage, and synovial CRP which took 3, 2, and 1 point, respectively. Patients with a total score of equal or greater than 6 were suggested infected, while a score between 2 and 5 was classified as inconclusive and required further intraoperative diagnostic score to fulfill the definition, and a score of 0 to 1 was defined as no infection.
The intraoperative diagnostic score consisted of positive histology, purulence, and a single positive culture which scored 3, 3, and 2 points, respectively. In combination with the inconclusive preoperative diagnostic score, patients with an overall score of equal or greater than 6 were considered infected, whereas a score between 4 and 5 was inconclusive and need further molecular findings, and a score of 3 or less was defined as aseptic (Table 2). The threshold of each laboratory test is detailed in Table 3. The sensitivity and specificity of this new scoring system are 97.7 and 99.5 percent, respectively, which is higher sensitivity than the previous diagnostic criteria [29].
| Major criteria (at least one of the following) | Decision | |
|---|---|---|
| Infected | |
| Minor criteria (Preoperative diagnosis) | Score | Decision |
Serum
| 2 1 | ≥6 Infected 2–5 Inconclusive (possibly infected)* 0–1 Not infected |
Synovial
| 3 3 2 1 | |
| Intraoperative diagnosis | Score | Decision |
Inconclusive preoperative score* or dry tap with
| 3 3 2 | ≥6 Infected 4–5 Inconclusive ≤3 Not infected |
Table 2.
The 2018 International Consensus Meeting on Musculoskeletal Infection scoring-based definition for PJI.
Modified from Parvizi et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33(5):1309–14.e2.
| Laboratory test | Acute (<90 days) | Chronic (>90 days) |
|---|---|---|
| 1. Serum CRP (mg/L) 2. Serum D-dimer (ng/mL) 3. Serum ESR (mm/h) 4. Synovial WBC count (cells/μL) 5. Synovial alpha-defensin (signal-to-cutoff ratio) 6. Synovial PMN (%) 7. Synovial CRP (mg/L) | 100 860 — 10,000 1 90 6.9 | 10 860 30 3,000 1 80 6.9 |
Table 3.
The threshold of laboratory test of the minor criteria.
Modified from Parvizi et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33(5):1309–14.e2.
3.2 Treatment
Management of PJI includes surgical intervention and medical treatment, especially antibiotics therapy, with the goals of eradicating the infection, minimizing pain by restoring the function of the infected joint before performing the revision arthroplasty, as well as reducing morbidity and mortality of the patients [30]. Tsukayama et al. classified characteristics of infection after TKA into four types with the guidance of surgical options among these scenarios (Table 4) [31].
| Type and definition | Characteristics | Treatment options |
|---|---|---|
| I: Positive intraoperative culture |
|
|
II: Early postoperative infection
|
|
|
|
|
|
| III: Acute hematogenous |
|
|
| IV: Late chronic |
|
|
Table 4.
Tsukayama classification and treatment options.
4. Periprosthetic fracture
Periprosthetic fracture after TKA is found increasingly in recent years due to a large number of performed TKAs and growing of geriatric population. This serious complication is impact to the quality of life and functional recovery of the patients, which is recognized to develop high morbidity and mortality [32]. The incidence of fracture following TKA varies from 0.3 to 5.5 percent in primary knee replacement and has been reported as high as 30 percent in revision knee surgery [33, 34]. The most common site of fracture is a supracondylar area of the distal femur which occurs ranging from 0.3 to 2.5 percent [32, 35], followed by patellar periprosthetic fracture, especially in the resurfaced patella, with an incidence around 0.68 percent. However, the true incidence of this type of fracture may be obscured from undetected and asymptomatic patients [36]. The least common pattern is a proximal tibial fracture which affected approximately 0.3 to 0.5 percent [37]. Most frequently, periprosthetic fracture results from low-energy trauma, and osteoporosis is considered a significant predictor of fracture risk [38]. The other predisposing factors are any causes that affected bone quality, for example, prolonged corticosteroid use, inflammatory joint diseases, especially rheumatoid arthritis, and patients with neurological and musculoskeletal problems, which have a high risk of falls [32, 35]. Iatrogenic causes from surgical procedure including anterior femoral notching, or alteration of anterior femoral cortex during bone preparation of distal femur is theorized to be an association of supracondylar femoral fracture after TKA [35]. A biomechanical study by Lesh et al. revealed a reduction of torsional strength and bending strength of the distal femur by 39.2 and 18 percent, respectively, after the full-thickness cortical defect was created [39]. However, the clinical outcome is still controversial [32, 40, 41]. The risk factors of periprosthetic tibial fractures in TKA are the use of long tibial stems, cementless press-fit fixation, malalignment of tibial component, and previous osteotomy of the tibia [37]. All other predisposing factors are detailed in Table 5 [42].
4.1 Classification
4.1.1 Femur
There were several classification systems described for supracondylar periprosthetic fracture of femur. Rorabeck et al. developed a classification that described fracture configuration and integrity of prosthesis to guide appropriate management of each fracture pattern. The key factors considered in the classification were the fracture displacement and the prosthesis stability [43, 44]. This classification later was widely known as “Lewis and Rorabeck classification” (Table 6).
| Medical factors | Surgical factors | ||
|---|---|---|---|
| Femur | Tibia | Patella | |
|
|
|
|
Table 5.
Predisposing factors associated with periprosthetic fractures after TKA.
| Type | Characteristics |
|---|---|
| I | Undisplaced fracture; prosthesis intact |
| II | Displaced fracture; prosthesis intact |
| III | Displaced or undisplaced fracture; prosthesis loosening or failing e.g., significant instability or polyethylene wear |
Table 6.
Lewis and Rorabeck classification.
The Lewis and Rorabeck classification recommended nonoperative treatment for type I classification [44]. However, Su et al. suggested surgical management in any type of fracture because of the high complication rate and further displacement in case of conservative treatment. An alternative classification was developed and proposed to characterize the fracture line in relation to the component for help in choosing among surgical options (Figure 1; Table 7) [45].
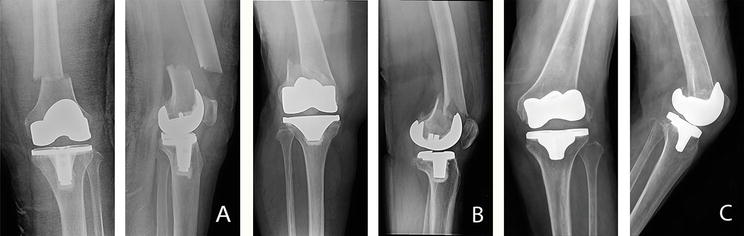
Figure 1.
Anteroposterior and lateral radiographs showing periprosthetic fracture of TKA (A) Su classification type I; Lewis and Rorabeck classification type II (B) Su classification type II; Lewis and Rorabeck classification type II (C) Su classification type III; Lewis and Rorabeck classification type III.
| Type | Characteristics |
|---|---|
| I | Fractures are proximal to the femoral component |
| II | Fractures originate at the proximal end of the component and extend proximally |
| III | Any part of the fracture line is distal to the upper edge of the component’s anterior flange |
Table 7.
Su classification.
4.1.2 Tibia
The Mayo classification described by Felix et al. (also known as Felix classification) is widely recognized to assess periprosthetic tibial fractures following TKA [46]. Fractures are classified into four types based on location and proximity to the prosthesis and each type is subcategorized by stability and whether the fracture occurred intraoperatively or postoperatively. The details are described in Table 8.
| Type | Characteristics |
|---|---|
| I | Fractures are located at the tibial plateau |
| II | Fractures occur inferior to the tibial plateau adjacent to the prosthetic stem |
| III | Fractures occur distal to the tibial stem |
| IV | Fractures involve the tibial tubercle |
| A | A fracture with a stable prosthesis on radiographs |
| B | Fractures with radiographic evidence of component loosening |
| C | Intraoperative fractures |
Table 8.
Mayo (Felix) classification.
4.1.3 Patella
The widely used classification for periprosthetic patellar fractures is the classification proposed by Goldberg et al. which is characterized by fracture configuration, stability of patellar component, and integrity of extensor mechanism [47]. The newer classification described by Ortiguera and Berry focused similarly on the stability of patellar components and integrity of extensor mechanism but differently on the quality of residual bone stock (Tables 9 and 10) [36].
| Type | Characteristics |
|---|---|
| I | Fractures are located in the periphery of the patella and do not involve the patellar component and the extensor mechanism |
| II | Fractures disrupt the implant-bone composite or the extensor mechanism |
III
| Fractures involve the inferior pole of the patella
|
| IV | Patellar fractures accompanied by patellofemoral dislocation |
Table 9.
Goldberg classification.
| Type | Characteristics |
|---|---|
| I | A stable implant and intact extensor mechanism |
| II | A stable implant with disruption of the extensor mechanism |
III
| Loose patellar component
|
Table 10.
Ortiguera and Berry classification.
4.2 Treatment
Fracture treatment options in each component are related on their classified types. For supracondylar femoral fracture, Lewis and Rorabeck classification recommended nonsurgical treatment in type I, whereas treatment options either closed reduction and fixation with an intramedullary nail or open reduction and internal fixation with a plate could be performed in type II. Type III fracture requires revision of the prosthesis using a long stem or structural allograft [44]. Su et al. suggested reduction with antegrade or retrograde intramedullary nail, or sometimes a fixed-angle device for Su classification type I fracture. Su classification type II requires management with either a fixed-angle device or retrograde supracondylar nail, and type III fracture may be managed with either a fixed-angle device or revision arthroplasty with a stemmed femoral component. However, if loosening is identified in any classification types, revision TKA with a femoral stem is recommended [45].
Felix et al. proposed a treatment algorithm for periprosthetic tibial fractures related to their classification. For type IA, nondisplaced IIA, and IIIA fracture, nonoperative treatment with protected weight-bearing is required. If displacement is observed in type IIA and IIIA fracture, closed reduction with casting or open reduction with internal fixation is recommended. Any loosening types (IB, IIB, and IIIB) should be treated with revision arthroplasty. In case of intraoperative fracture (subcategory C), bracing with protected weight-bearing can be treated in any type if the fracture is stable and nondisplaced. However, in unstable fracture pattern or displaced fracture, further surgical management is required. Type IC fracture may be treated by screw fixation and/or a long-stemmed tibial prosthesis to bypass the fracture site. Type IIC fracture can be managed with bone grafting at the cortical defect and bypassing the fracture site with a long tibial stem. Type IIIC fracture can be treated with either closed reduction and casting or open reduction with internal fixation [46].
For treatment of patellar periprosthetic fracture, Ortiguera and Berry suggested nonoperative treatment for type I fracture. If patients developed extensor mechanism disruption with a well-fixed implant (type II), open reduction with internal fixation of the displaced fragment, or alternatively, patellectomy with advancement and repair of the extensor mechanism is recommended. Operative treatment for type IIIA fracture required revision of the patellar component or component resection with patelloplasty, whereas implant removal with patellectomy is recommended for type IIIB fracture [36].
In the elderly, physiologic changes of bone, especially a high rate of bone resorption, result in diminishing bone mass and strength [48]. Osteoporosis workup and treatment are necessary in addition to fracture management in patients with periprosthetic fracture after TKA.
5. Conclusions
This chapter concludes with the principle, classification, and management of three typical conditions, which are considered serious and unsatisfied results after TKA. Causes of stiff TKA divide into three different periods and each period needs specific management, but the most important risk factor for postoperative stiffness is the limitation of preoperative ROM. Patient education and motivation either before or after surgery are necessary to prevent further problems and meet the patient’s satisfaction. An exploration of new diagnostic tests enhances the accuracy of PJI diagnosis and the latest scoring-based definition achieved more sensitivity than the previous criteria. Major criteria of two positive cultures of a similar pathogen or the presence of a sinus tract to the knee joint can diagnose PJI. If a diagnosis has not been made, the further investigation of minor criteria, including serum and synovial laboratory tests, would have been collected preoperatively. An inconclusive diagnosis from the minor criteria needs furthermore investigation from intraoperative findings. Periprosthetic fractures are principally classified from the anatomy of fracture site. The most common is a femoral supracondylar fracture and the surgical options depend on fracture location and configuration. Finally, the goal of treatment among these three conditions is return to ambulation with a well-function knee prosthesis.
References
- 1.
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet. 2012; 379 (9823):1331-1340 - 2.
Gunaratne R, Pratt DN, Banda J, Fick DP, Khan RJK, Robertson BW. Patient dissatisfaction following total knee arthroplasty: A systematic review of the literature. The Journal of Arthroplasty. 2017; 32 (12):3854-3860 - 3.
Healy WL, Della Valle CJ, Iorio R, Berend KR, Cushner FD, Dalury DF, et al. Complications of total knee arthroplasty: Standardized list and definitions of the Knee Society. Clinical Orthopaedics and Related Research. 2013; 471 (1):215-220 - 4.
Manrique J, Gomez MM, Parvizi J. Stiffness after total knee arthroplasty. The Journal of Knee Surgery. 2015; 28 (2):119-126 - 5.
Choi HR, Siliski J, Malchau H, Freiberg A, Rubash H, Kwon YM. How often is functional range of motion obtained by manipulation for stiff total knee arthroplasty? International Orthopaedics. 2014; 38 (8):1641-1645 - 6.
Laubenthal KN, Smidt GL, Kettelkamp DB. A quantitative analysis of knee motion during activities of daily living. Physical Therapy. 1972; 52 :34-43 - 7.
Fitzsimmons SE, Vazquez EA, Bronson MJ. How to treat the stiff total knee arthroplasty?: A systematic review. Clinical Orthopaedics and Related Research. 2010; 468 (4):1096-1106 - 8.
Chareoncholvanich K. Complications after Total Knee Arthroplasty. In: Charoencholvanich K, editor. Reconstructive Surgery of the Osteoarthritic Knee. Bangkok: Faculty of Medicine Siriraj Hospital, Mahidol University; 2016. pp. 283-319 - 9.
Schiavone Panni A, Cerciello S, Vasso M, Tartarone M. Stiffness in total knee arthroplasty. Journal of Orthopaedics and Traumatology. 2009; 10 (3):111-118 - 10.
Lizaur A, Marco L, Cebrian R. Preoperative factors influencing the range of movement after total knee arthroplasty for severe osteoarthritis. The Journal of Bone and Joint Surgery. British Volume (London). 1997; 79 (4):626-629 - 11.
Lee SA, Kang SB, Chang CB, Chang MJ, Kim YJ, Song MK, et al. Does the severity or cause of preoperative stiffness affect the clinical results and range of motion after total knee arthroplasty? PLoS One. 2018; 13 (10):e0205168 - 12.
Pua YH, Poon CL, Seah FJ, Thumboo J, Clark RA, Tan MH, et al. Predicting individual knee range of motion, knee pain, and walking limitation outcomes following total knee arthroplasty. Acta Orthopaedica. 2019; 90 (2):179-186 - 13.
Järvenpää J, Kettunen J, Soininvaara T, Miettinen H, Kröger H. Obesity has a negative impact on clinical outcome after total knee arthroplasty. Scandinavian Journal of Surgery. 2012; 101 (3):198-203 - 14.
Bong MR, Di Cesare PE. Stiffness after total knee arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2004; 12 (3):164-171 - 15.
Laoruengthana A, Jarusriwanna A, Rattanaprichavej P, Rasamimongkol S, Varakornpipat P, Pongpirul K. Timing of periarticular injection has no effect on postoperative pain and functional recovery in simultaneous bilateral total knee arthroplasty: A prospective randomized, double-blinded trial. BMC Musculoskeletal Disorders. 2019; 20 (1):162 - 16.
Cheuy VA, Foran JRH, Paxton RJ, Bade MJ, Zeni JA, Stevens-Lapsley JE. Arthrofibrosis associated with total knee arthroplasty. The Journal of Arthroplasty. 2017; 32 (8):2604-2611 - 17.
Freeman TA, Parvizi J, Dela Valle CJ, Steinbeck MJ. Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty. Fibrogenesis & Tissue Repair. 2010; 3 :17 - 18.
Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis & Tissue Repair. 2009; 2 (1):5 - 19.
Maloney WJ. The stiff total knee arthroplasty: Evaluation and management. The Journal of Arthroplasty. 2002; 17 (4 Suppl. 1):71-73 - 20.
Stamos VP, Bono JV. Management of the stiff total knee arthroplasty. In: Bono JV, Scott RD, editors. Revision Total Knee Arthroplasty. New York: Springer; 2005. pp. 251-257 - 21.
Goswami K, Parvizi J, Maxwell CP. Current recommendations for the diagnosis of acute and chronic PJI for hip and knee-cell counts, alpha-defensin, leukocyte esterase, next-generation sequencing. Current Review Musculoskeletal Medicine. 2018; 11 (3):428-438 - 22.
Lenguerrand E, Whitehouse MR, Beswick AD, Toms AD, Porter ML, Blom AW, et al. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: An analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open. 2017; 7 (7):e014056 - 23.
Mortazavi SM, Schwartzenberger J, Austin MS, Purtill JJ, Parvizi J. Revision total knee arthroplasty infection: Incidence and predictors. Clinical Orthopaedics and Related Research. 2010; 468 (8):2052-2059 - 24.
Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. INFORM Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS One. 2016; 11 (3):e0150866 - 25.
Rosteius T, Jansen O, Fehmer T, Baecker H, Citak M, Schildhauer TA, et al. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. Journal of Medical Microbiology. 2018; 67 (11):1608-1613 - 26.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clinical Orthopaedics and Related Research. 2011; 469 (11):2992-2994 - 27.
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2013; 56 (1):1-10 - 28.
Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint Journal. 2013; 95 (11):1450-1452 - 29.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. The Journal of Arthroplasty. 2018; 33 (5):1309-14.e2 - 30.
Tande AJ, Patel R. Prosthetic joint infection. Clinical Microbiology Reviews. 2014; 27 (2):302-345 - 31.
Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. The Journal of Bone and Joint Surgery. American Volume. 2003; 85-A :S75-S80 - 32.
Whitehouse MR, Mehendale S. Periprosthetic fractures around the knee: Current concepts and advances in management. Current Reviews in Musculoskeletal Medicine. 2014; 7 (2):136-144 - 33.
Capone A, Congia S, Civinini R, Marongiu G. Periprosthetic fractures: Epidemiology and current treatment. Clinical Cases in Mineral and Bone Metabolism. 2017; 14 (2):189-196 - 34.
Della Rocca GJ, Leung KS, Pape HC. Periprosthetic fractures: Epidemiology and future projections. Journal of Orthopaedic Trauma. 2011; 25 (Suppl. 2):S66-S70 - 35.
Yoo JD, Kim NK. Periprosthetic fractures following total knee arthroplasty. Knee Surgery Related Research. 2015; 27 (1):1-9 - 36.
Ortiguera CJ, Berry DJ. Patellar fracture after total knee arthroplasty. The Journal of Bone and Joint Surgery. American Volume 2002; 84 (4):532-540 - 37.
Schreiner AJ, Schmidutz F, Ateschrang A, Ihle C, Stöckle U, Ochs BG, et al. Periprosthetic tibial fractures in total knee arthroplasty - an outcome analysis of a challenging and underreported surgical issue. BMC Musculoskeletal Disorders. 2018; 19 (1):323 - 38.
Rhee SJ, Cho JY, Choi YY, Sawaguchi T, Suh JT. Femoral periprosthetic fractures after total knee arthroplasty: New surgically oriented classification with a review of current treatments. Knee Surgery Related Research. 2018; 30 (4):284-292 - 39.
Lesh ML, Schneider DJ, Deol G, Davis B, Jacobs CR, Pellegrini VD Jr. The consequences of anterior femoral notching in total knee arthroplasty. A biomechanical study. The Journal of Bone and Joint Surgery. American Volume. 2000; 82 (8):1096-1101 - 40.
Ritter MA, Thong AE, Keating EM, Faris PM, Meding JB, Berend ME, et al. The effect of femoral notching during total knee arthroplasty on the prevalence of postoperative femoral fractures and on clinical outcome. The Journal of Bone and Joint Surgery. American Volume. 2005; 87 (11):2411-2414 - 41.
Gujarathi N, Putti AB, Abboud RJ, MacLean JG, Espley AJ, Kellett CF. Risk of periprosthetic fracture after anterior femoral notching. Acta Orthopaedica. 2009; 80 (5):553-556 - 42.
Sarmah SS, Patel S, Reading G, El-Husseiny M, Douglas S, Haddad FS. Periprosthetic fractures around total knee arthroplasty. Annals of the Royal College of Surgeons of England. 2012; 94 (5):302-307 - 43.
Rorabeck CH, Angliss RD, Lewis PL. Fractures of the femur, tibia, and patella after total knee arthroplasty: Decision making and principles of management. Instructional Course Lectures. 1998; 47 :449-458 - 44.
Rorabeck CH, Taylor JW. Classification of periprosthetic fractures complicating total knee arthroplasty. The Orthopedic Clinics of North America. 1999; 30 (2):209-214 - 45.
Su ET, DeWal H, Di Cesare PE. Periprosthetic femoral fractures above total knee replacements. The Journal of the American Academy of Orthopaedic Surgeons. 2004; 12 (1):12-20 - 46.
Felix NA, Stuart MJ, Hanssen AD. Periprosthetic fractures of the tibia associated with total knee arthroplasty. Clinical Orthopaedics and Related Research. 1997; 345 :113-124 - 47.
Goldberg VM, Figgie HE 3rd, Inglis AE, Figgie MP, Sobel M, Kelly M, et al. Patellar fracture type and prognosis in condylar total knee arthroplasty. Clinical Orthopaedics and Related Research. 1988; 236 :115-122 - 48.
Unnanuntana A, Jarusriwanna A, Vanitcharoenkul E. Bone physiology and age-related bone changes. In: Unnanuntana A, editor. Textbook of Hip Fracture in The Elderly. Bangkok: Faculty of Medicine Siriraj Hospital, Mahidol University; 2019. pp. 18-39