Abstract
Primary amines are combined with an aldehyde group to generate Schiff base compounds, which are called condensation imine products. This class of compounds has a general structure, R-C=NR\', where R and R\' represent alkyl/aryl/cyclohexyl/heterocyclic group. These compounds contain an azomethine group that is basic in nature due to, (i) the presence of lone pair of electrons on the nitrogen and (ii) electron-donating nature of the double bond. Hence, these compounds, as ligands, participate in the formation of metal complexes. The presence of lone pair of electrons on the nitrogen atom and the hybridization involved explains the physical, chemical, and spectral properties of nitrogen-containing moieties. In the case of (sp2) hybridization (trigonal structure), the lone pair of electrons occupies either a symmetrical unhybridized 2p orbital that is perpendicular to the plane of trigonal hybrids or a symmetrical hybrid orbital, whose axis is in the plane, leaving behind only the π-electrons in the unhybridized 2p orbital. A very similar type of hybridization is experienced by the nitrogen atom in the azomethine group. Traditional phosphine complexes of nickel, palladium, and platinum, particularly those of palladium, have played an extremely important role in the development of homogeneous catalysis. Schiff base complexes as catalysts have been studied for various organic transformations such as oxidation, epoxidation, reduction, coupling reactions, polymerization reactions, hydroformylations, and many more.
Keywords
- Schiff base metal-complexes
- homogenous catalysis
- oxidation
- C-C coupling
- epoxidation
1. Introduction
Schiff base ligands (SBLs) are known as “privileged ligands” [1] due to their facile synthesis by the condensation of aldehydes with imines. In 1864, Hugo Schiff first introduced the condensation of an aldehyde and an amine, which leads to the formation of a Schiff base (SB) [2]. SBLs are capable to form coordination bonds with metals
The basic structural formula for SBs is RRC=N-R, where R, R’, here R is labeled as hydroxyalkyl, alkyl, cyclohexyl, hydroxyaryl, etc. SBs consist of azomethine moiety (C=N) an azomethine group. In some cases, -SH or –OH functional groups also participate in complexation, when they are enough close to azomethine moiety. This is due to the lone pair of electron’s being donated to the electron deficient metal ion. Overall, this facilitates the development of a metal complex with good stability. With salicylideneimine, Ettling created the copper (II) complex in 1840. Since then, there has been a significant quantity of material published on SBs and related metal complexes (MCs) [6].
2. Synthetic routes to Schiff base complexes
From the past two decades, there is significant progress in the formation of SB transition MCs, with an increased focus on synthesis and structure. The characteristic of the SB and metal ion determines the synthesis, characteristics, and structure of the SBCs. Mostly, the following techniques have been employed to create MCs of SBs [7].
Aldehydes and amines can condense with one another under various reaction circumstances in the presence of various solvents. Methanol and ethanol are the typical solvents used to prepare the SBs, either at room temperature or under refluxing conditions. SBs are typically more likely to form when dehydrating chemicals such as magnesium sulfate are present. If the syntheses are conducted in organic solvents, the water generated during the reaction can be extracted easily. During the purification process, the SBs may deteriorate. It is preferable in these circumstances to crystallize the SBs and purify them. Here, in a suitable solvent, SB is permitted to interact with metal ions. The use of a binary azeotropic mixture of water and an organic solvent has been documented in some situations, and using an organic solvent is recommended to prevent the hydrolysis of the azomethine group.
2.1 Direct synthesis
Here, a suitable solvent is used to facilitate the reaction between SB and metal ions. Despite the fact that there have been instances of mixing organic solvent and water, the use of organic solvent is recommended to prevent the hydrolysis of the azomethine group [8].
2.2 In situ method
This procedure offers the addition of the metal ion during or immediately after the aldehyde and amine have reacted with each other. Metal ions have been found to typically pair with just one element. Before other components are added, the reaction speeds up toward the formation of the complex [9].
2.3 Coordinated secondary amine oxidation
For this kind, the secondary amine’s oxidative dehydrogenation is facilitated by the metal ion followed by the direct formation of Schiff base complexes. In Figure 1, one such response is provided.
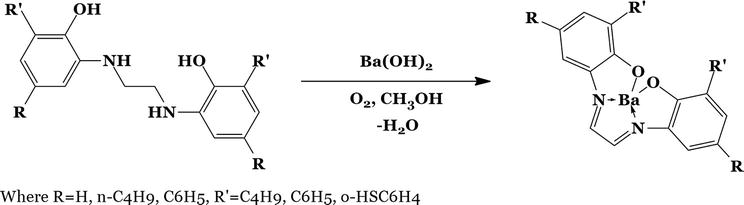
Figure 1.
Synthesis of SB from a secondary amine.
2.4 Amine exchange reaction
This process involves the reaction of an amine and a transition metal complex of SBs, which results in the exchange of aryl and alkyl groups as given below [10].
Where: R =
2.5 Metal exchange reaction
This is an interesting route for the preparation of simple metal to carbon bond. The sample below shows a common metal interchange reaction [10].
2.6 Ligand exchange reaction
It is very challenging to synthesize molecules such as dioxomolybdenum(VI) complexes, for which there are different techniques. The most preferred method is the ligand exchange method. Below is an example of a common response [11].
Where: acac = acetylacetone.
2.7 Template synthesis
A template reaction in coordination chemistry is any of a group of ligand-based reactions that take place between two or more nearby coordination sites on a metal center. When there is no metal ion present, the same organic reactants result in different end products. Although the coordination modifies the electronic properties of ligands (acidity, electrophilicity, etc.), the template effects highlight the pre-organization provided by the coordination sphere. In a general sense, transition metal-based catalysis can be viewed as template reactions: Reactants coordinate to adjacent sites on the metal ion and, owing to their adjacency, the two reactants interconnect (insert or couple) either directly or
This process involves the interaction of an aldehyde, an amine, and a metal compound in a single step to create complexes without the need to isolate Schiff bases [12, 13]. Metal ions are serving as a catalyst for these reactions. Rotaxanes, helicates, macro cycles, and catenanes are just a few examples of assemblies with unique topologies that have been produced using template synthesis [14]. The information needed to arrange a group of building blocks so that they can be connected in a certain way is therefore said to be contained in a templating agent. Thermodynamic and kinetic processes are the two different categories of these templates. The template binds to one of the reactants in the first case, shifting the equilibrium in favor of the product formation. With regard to kinetic processes, the templates work under conditions that are irreversible, stabilizing each transition state and causing the formation of the intended product. The template is tightly coupled to the end species in many kinetically regulated processes. In these situations, it serves as both a thermodynamic template and a kinetic template. Gimeno et al. defined a template can be class that arranges a grouping of molecular construction pieces through noncovalent interactions that favor the creation of a particular complex in their review (Figure 2) [15].
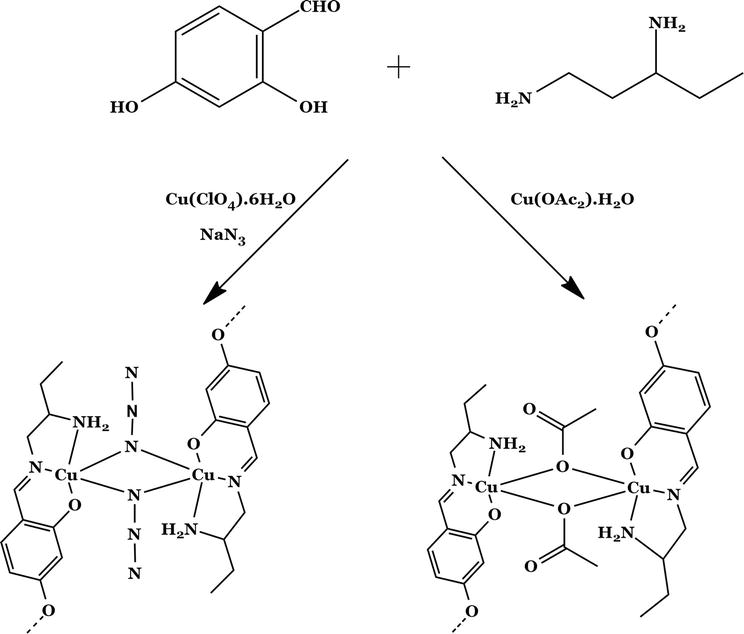
Figure 2.
Bridged dinuclear copper (II) SB complex using template method.
2.8 Rearrangement of heterocycles (thiazole and oxazoles)
When an
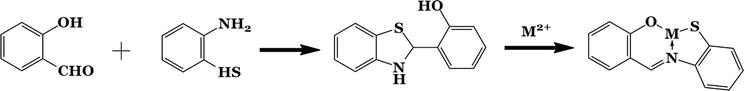
Figure 3.
2-(2-hydroxyphenyl) benzothiazoline.
3. Chemistry of Schiff bases
Hugo Schiff, a Nobel Prize laureate, first identified SBs as a condensation outcome of carbonyl compounds and primary amines [18]. SB is a structural counterpart of an –CHO or –C=O where C=O has been exchanged out for an imine or an azomethine group (Figure 4) [3, 19].
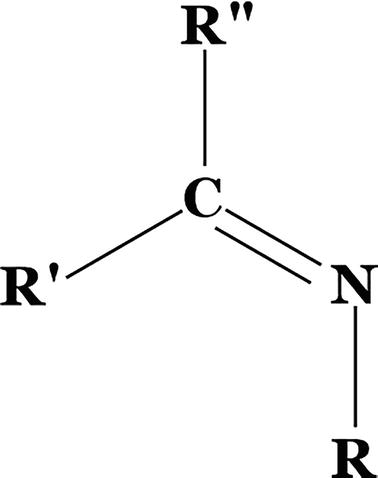
Figure 4.
Schiff base.
A chemical molecule known as a Schiff Base, or SB, contains a C-N double bond, where the N is linked to an aryl group or an alkyl (R) but, not to an H-atom. A molecule containing azomethine linkage is the same as the SB. Hugo Schiff was honored by having his name attached to several compounds, which generally have the following structure:
The SB is an imine since R stands for an alkyl or phenyl group, making it a stable compound. Even the imine N and other group, typically connected with aldehyde, this type of ligands, can coordinate metal ions. SBs are still created by chemists, and today’s “privileged ligands” are active, well-designed Schiff base ligands [20]. The bridging SBs possess below given structure, where it consists of several functional groups that can be altered depending on the situation (Figure 5).
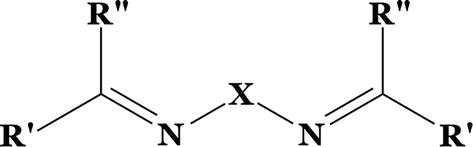
Figure 5.
Bridged SB.
Where R’ here might be a H or R group, R” is a phenyl or substituted phenyl, and X is alkyl or aryl group. In fact, SBs can influence the activity of metals in a broad range of beneficial reactions in catalysis by stabilizing a wide variety of metals in a variety of oxidation states [21]. The oxygen atoms in SBs are often donated by NO or N2O2, although they can be substituted by S, N, or Se atoms. Figure 6 shows the synthesis of SB by condensation reaction.

Figure 6.
Synthesis of SB by condensation reaction.
R, here, might be either aryl or an alkyl group. Aryl SBs are significantly more stable and easier to synthesize than their alkyl counterparts, which are more prone to instability. Aliphatic aldehyde SBs are comparatively not stable and easily undergo polymerization [22]. Aldehydes or ketones can be converted into SBs in a reversible process that often occurs when the substance is heated or when an acid or a base catalyzes the reaction (Figure 7).
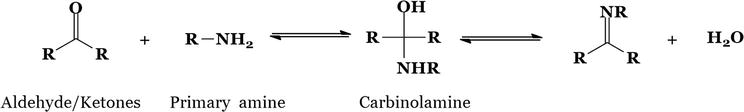
Figure 7.
Reversible reaction of SB formed by the interaction of aldehydes or ketones.
In most cases, the product is separated from the reaction, or when the water formed is removed, or sometimes in both cases. Aqueous acid or base has the ability to hydrolyze several Schiff bases back to their respective aldehydes, ketones, and amines. Nucleophilic addition to the carbonyl group is a common theme in the synthetic route of Schiff base formation. The amine serves as a nucleophile during this instance. In the first step of the procedure, the amine reacts with the aldehyde or ketone to produce an unstable addition substance known as carbinolamine. Either an acid- or a base-catalyzed pathway results in the carbinolamine losing water. This carbinolamine undergoes acid-catalyzed dehydration since it is an alcohol (Figure 8).

Figure 8.
Mechanism of formation SB.
Usually, the rate-determining step in the creation of a SB is dehydration of the carbinolamine, which is why acids are used to catalyze the reaction. Yet because amines are basic chemicals, the acid concentration should not be exceeded. Equilibrium shifts to the left, and carbinolamine production is prevented if the amine is protonated and turns non-neutral. As a result, it is ideal to synthesize numerous Schiff bases at a pH that is a little acidic. The base also acts as a catalyst for the dehydration of carbinolamines. It goes through an anionic intermediate in two phases. In reality, the SB creation is a series of two different kinds of reactions, namely addition and elimination [23].
4. SB complexes in catalysis
Excellent catalytic activity is displayed by several SBCs in diverse reactions and in the presence of moisture. Numerous studies on their use in homogeneous and heterogeneous catalysis have been published during the past few years. The high heat and moisture stabilities of many Schiff base complexes proved helpful for their application as catalysts in high-temperature processes. Due to the fact that complexation often causes an increase in activity, knowledge of the characteristics of both ligands and metals can help in the synthesis of substances that have high activity [14].
SBs were treated as crucial ligands in coordination chemistry for a long time [24, 25]. SBs are an essential type of ligands in coordination chemistry [26, 27, 28]. Owing to their relatively straightforward synthesis and diverse structural makeup, SBs and their metal complexes were extensively studied because of their extraordinary properties and uses in variety of fields. These compounds are revealed to be auspicious for the synthetic and structural research field [1, 29, 30].
From the finding of chiral Mg (III) salen SB catalysts in the asymmetric epoxidation of unfunctionalized olefins in the 1990s, the tetradentate SB, salen-type ligand, and their complexes have attracted significant attention [31]. Due to their potential application as catalysts in a variety of C-C and C-N bond formation events, such as cyclopropanations, aziridination, asymmetric cycloaddition reactions, and A3-coupling, among others, Salen-Schiff base transition MCs have been well studied (Figure 9 [32, 33]. For medicinal chemists, the design of the C-N bonds of aryl compounds is extremely crucial. Tetrazoles are a significant group of heterocycles among the many heterocycles that have been reported. In coordination chemistry, tetrazoles have been utilized as ligands, [34] stabilizers in the photographic industry, [35] linkers to covalently bind synthetic groups to biopolymers in a specific manner, etc. [36]. The A3-coupling reaction is an appealing illustration of a multi-component reaction [37]. (Figure 10) [38]. Agrahari and coworkers carried out an A3-coupling reaction with less use of catalyst, and the catalyst was reclaimed for four successive cycles in the reactions [39].
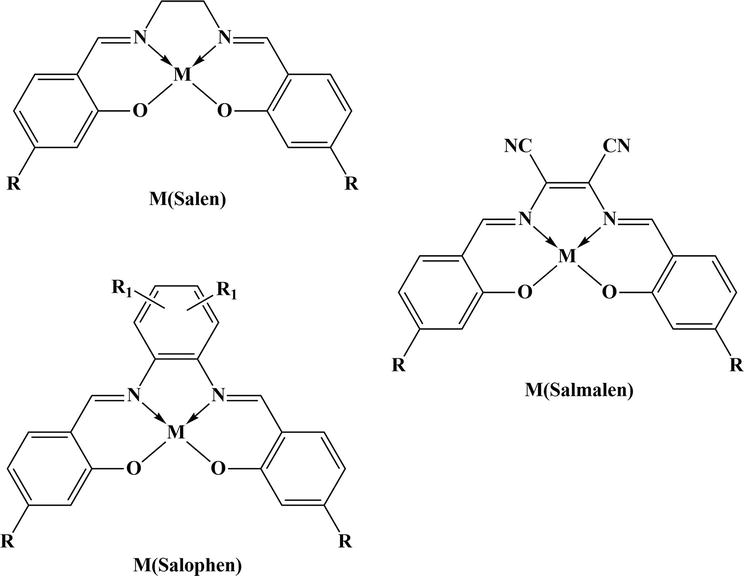
Figure 9.
Metal salen-type complexes.
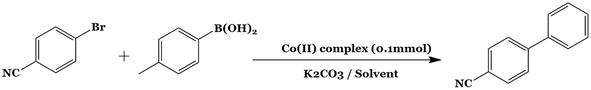
Figure 10.
4-bromobenzonitrile with phenylboranic acid.
The primary method for producing biaryls is the Suzuki-Miyaura cross-coupling reaction. This reaction is an effective technique for the production of medicines due to the properties and convenient accessibility of organoboron reagents [40]. Palladium is one of the transition metals that are most frequently employed in both industry and academia to produce C-N, C-S, and C-C bonds because it is a catalytically active metal [41]. In this context, during the past few decades, significant attempts have been made to synthesize potential substitute catalysts for cross-coupling reactions [42]. Transition metals in the first row are a particularly promising class of molecules that have the potential to be more useful in the field of catalysis than other transition MCs [43]. This work deals with the less harmful alternative to the commonly utilized palladium-phosphine ligand complexes for initiating Suzuki reactions: SB-Cobalt complexes. This work describes the synthesis of OON- and ONN-SBLs and their Co complexes. The created compounds were evaluated as Suzuki coupling reaction catalysts. This work utilized only 0.1 mM catalyst for the reaction [44].
SBL, BCNPOH, and metal salts (Cu, Co, Ni, Fe, and Cr) were individually dissolved in ethanol and followed by mixing both the solutions and reflux for 5 h at 70°C. The obtained precipitate was filtered and dried. CuL, CoL, NiL (Figure 10, Scheme 1b), FeL, and CrL (Figure 11, Scheme 1c) produced SB complexes that were recrystallized in ethanol [45].

Figure 11.
Scheme 1(a–c) synthetic method for the preparation of Schiff base complex of BCNPOH and metal oxides.
The synthesized complexes were implemented for the C–C coupling reaction of Phenylboronic Acid and Iodobenzene and Phenylboronic Acid and 2,6-Dibromopyridine.
The coupling reaction between iodobenzene and phenylboronic acid was conducted (Figure 12). The reaction was completed in 95:5 DMF:water mixture under an inert nitrogen environment.
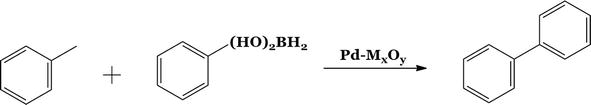
Figure 12.
Scheme3: C-C coupling reaction of phenylboronic acid and iodobenzene.
The coupling reaction between 2,6-dibromopyridine and phenylboronic acid was assessed at 110°C for 24 hours. (Figure 13). The reaction was taken to react in 5 mL of 95:5 DMF:water combination under an inert nitrogen environment with the limiting reagent 2,6-dibromopyridine and a molar ratio of the halide to the boronic acid of 1:2.4. The amount of catalyst was always 25 mg, and the molar ratio of the base (K2CO3) to the halide was 1:2 [45].
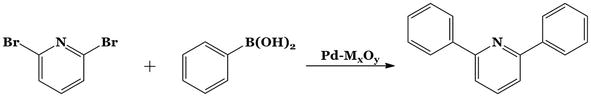
Figure 13.
C-C coupling reaction of phenylboronic acid and 2, 6-dibromopyridine.
5. Synthesis for the transfer hydrogenation of ketones
Through the use of pre-catalyst Ru(II) (Figure 14), the catalytic transfer hydrogenations of ketones were investigated (0.001 mmol). In
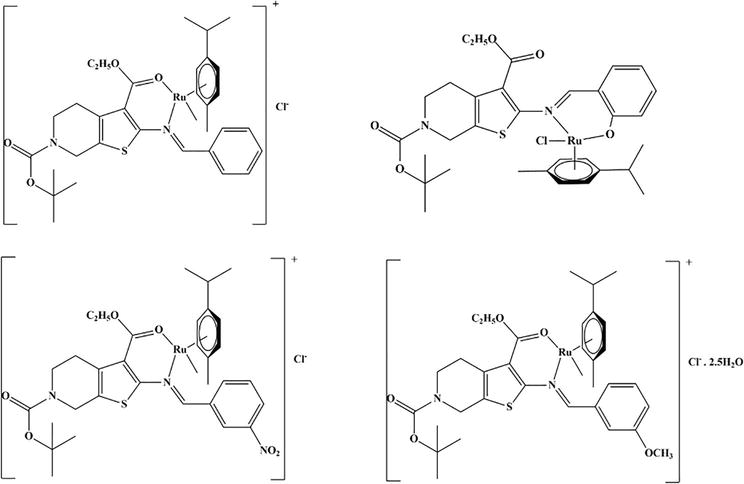
Figure 14.
Structures of Ru(II) pre-catalyst.
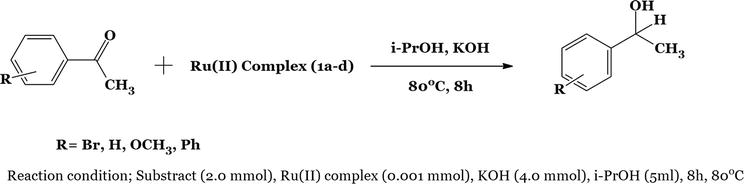
Figure 15.
Hydrogenation of ketone using Ru(II).
Reliable zinc complexes for bulk polymerization of lactide have been studied at 150°C under applicable commercial circumstances. The utilized anionic Schiff base ligands (Figure 14) may be produced aerobically and are extremely stable against air, moisture, and other lactide contaminants. As a result, these compounds incorporate both a strong anionic ligand and a nontoxic, active zinc core (Figure 16). These compounds are ideally suited for lactide polymerization under industrially relevant circumstances due to their simple aerobic synthesis, high thermal stability under nitrogen, and air [47].
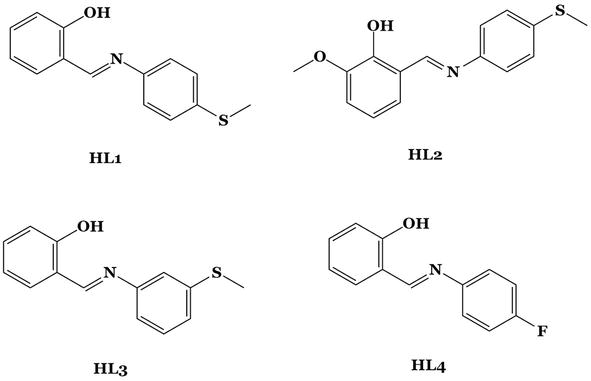
Figure 16.
SBLs HL1–HL4, used for the synthesis of four new zinc Schiff base complexes.
Cu-SBCs based on particularly ordered mesoporous silica MCM-41 were synthesized by Niakan et al., to create a heterogeneous catalyst (Figure 17). The synthesized catalyst was implemented for coupling reactions of Ullmann type. The synthesized catalyst showed excellent activity for the N-arylation of amines with aryl iodides and bromides. Here, 0.8 mol% of catalyst was used [48].
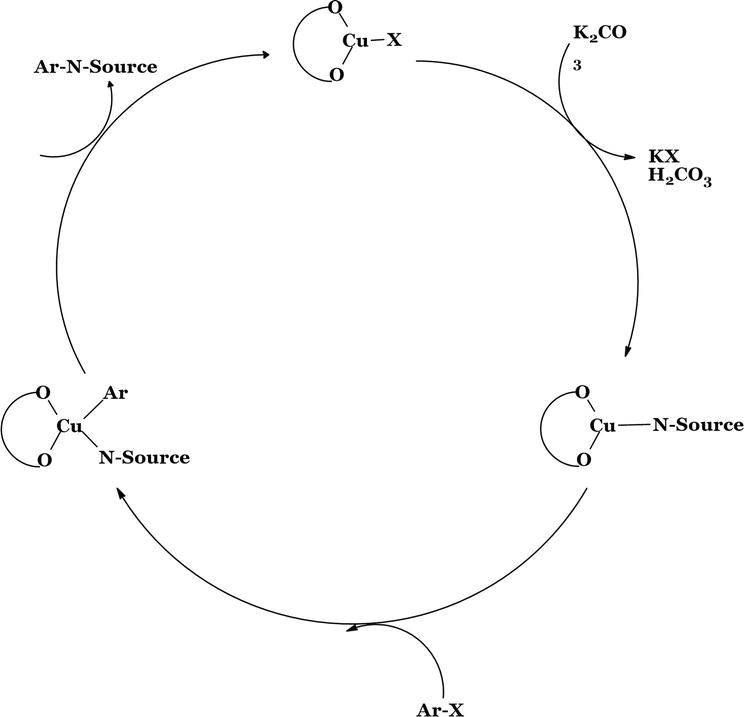
Figure 17.
A plausible mechanism for the Cu-SB-MCM-41-catalyzed C-N bond formation reaction.
Bunno and group synthesized metal-containing SB/ Sulfoxides that served as chiral ligands for asymmetric intramolecular allylic C-H amination processes that are catalyzed by Pd(II).They claimed that the use of metal-containing Schiff base ligands helped to tune the reaction conditions. In the reaction, internal and terminal alkenes were both utilized [49]. A novel Cu(II) SBC on graphene oxide, GO-SB-Cu was successfully synthesized through surface functionalization of the GO (Figure 18). Potential of the catalyst was tested as heterogeneous catalyst in one-pot three component click reactions for synthesis of 2H-indazoles and 1,4-disubstituted 1,2,3-triazoles (Figure 19).
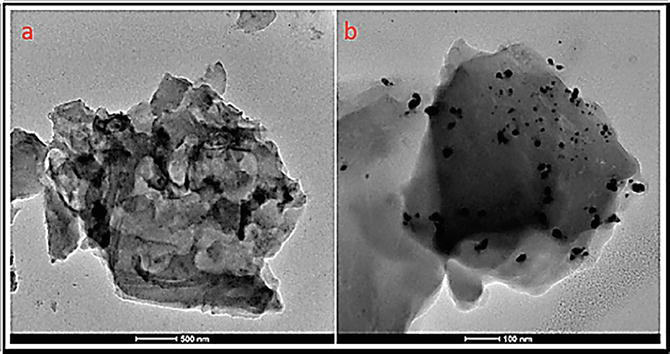
Figure 18.
HRTEM images of GO-SB-Cu.

Figure 19.
Synthesis of 2H-indazole.
A reaction was carried out with aniline and 2-bromobenzaldehyde in the presence of sodium azide. Different reactions were carried out to set the optimized conditions using catalyst loading, solvent selectivity, temperature, and time for catalytic study of GO-SB [50].
One more important catalytic conversion reaction is in oxidation of alcohols into carbonyl compounds. A cobalt (II)-SBC with triphenylphosphine was synthesized (Figure 20). The catalyst was used for oxidation reactions using different substrates and obtained good % of conversion of carbonyl compound [51]. Dileep et al. have done experiment on the oxidation of primary and secondary alcohols to respective carbonyl compounds
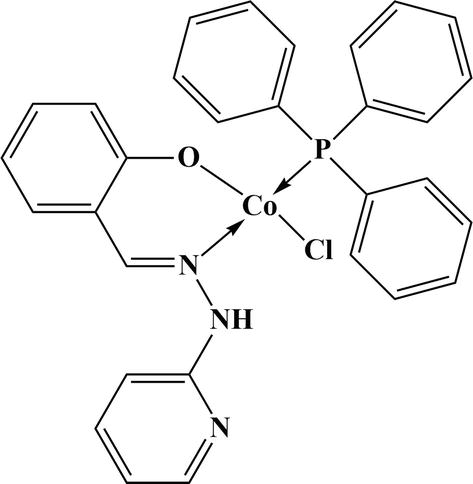
Figure 20.
Cobalt (II)-SBC with triphenylphosphine.
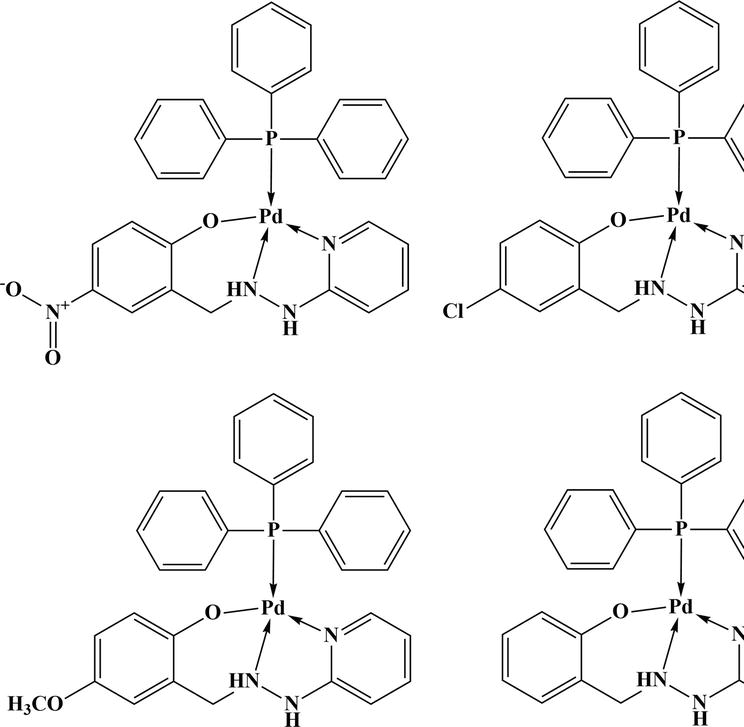
Figure 21.
Palladium SBC with triphenylphosphine.
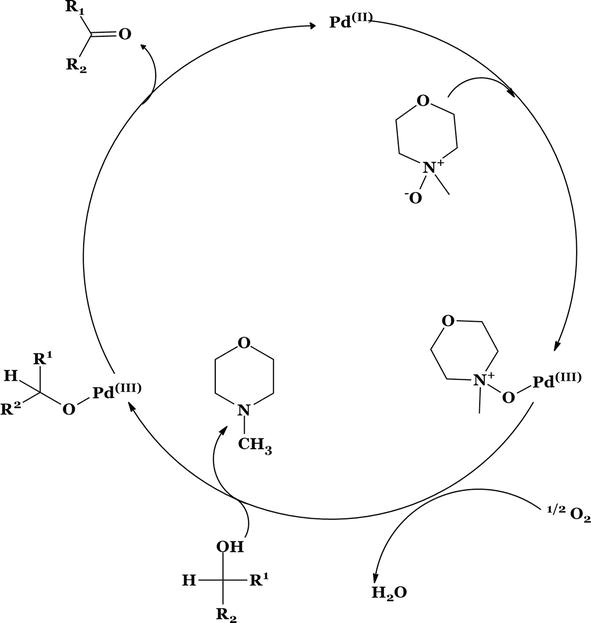
Figure 22.
Proposed mechanism for palladium complex catalyzed oxidation of alcohols by NMO.
A novel Ru-SBC composed of Ph3P was synthesized by and characterized by various techniques. The synthesized complexes were implemented for the oxidation of alcohols in [EMIM]Cl ionic liquid-NaOCl system. Recycling of catalyst is an important factor in catalysis. Herein, recyclability of catalyst for the conversion of benzylalcohol is carried till seven consecutive cycles, shown in Figure 23. Recyclability graph indicates that recycling of ionic liquid has small significant effect on the conversion of the product [54].
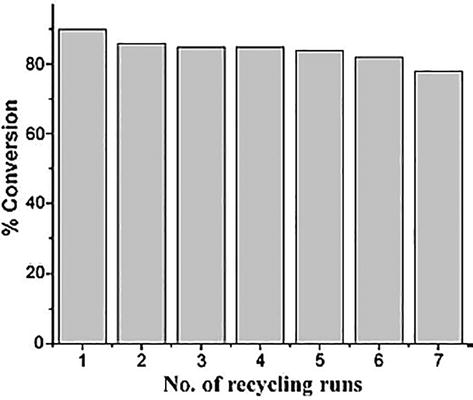
Figure 23.
Results of recycling on conversion of benzylalcohol.
For the catalytic oxidation of primary and secondary alcohols with periodic acid (H5IO6) as the oxidant, novel copper complexes were created and put into use [55]. Palladium was used as a catalyst in the carbonylation of alcohols in ionic liquid (IL) media (1-ethyl-3-methylimidazolium hexafluorophosphate). The usage of Pd-based catalyst, where NaOCl is utilized as an oxidant, considerably improved the carbonylation of primary/secondary alcohols to aldehydes/ketones [56].
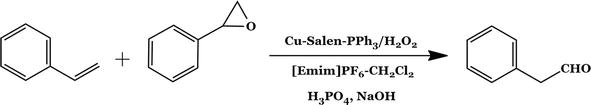
Figure 24.
Scheme for epoxidation of alkenes.
A SB Pd complex was studied for the catalytic activity in ethyl-methyl imidazolium hexafluorophosphate [EMIM] PF6 ionic liquid. This work shows air stable system for Heck and Suzuki reactions. In the [EMIM] PF6-Water system, the palladium-catalyzed coupling process demonstrated remarkable fidelity [58]. Copper complex was studied for the catalytic application in Heck and Suzuki reactions (Figure 25). The reactions were conducted in [EMIM]PF6 ionic liquid-water mixture with 1:1 ratio, which was observed to be helpful during catalyst recycling and reusing [59]. These compounds demonstrated improved catalysis for the N-alkylation step required to produce benzazoles (Figure 26). The reaction progressed
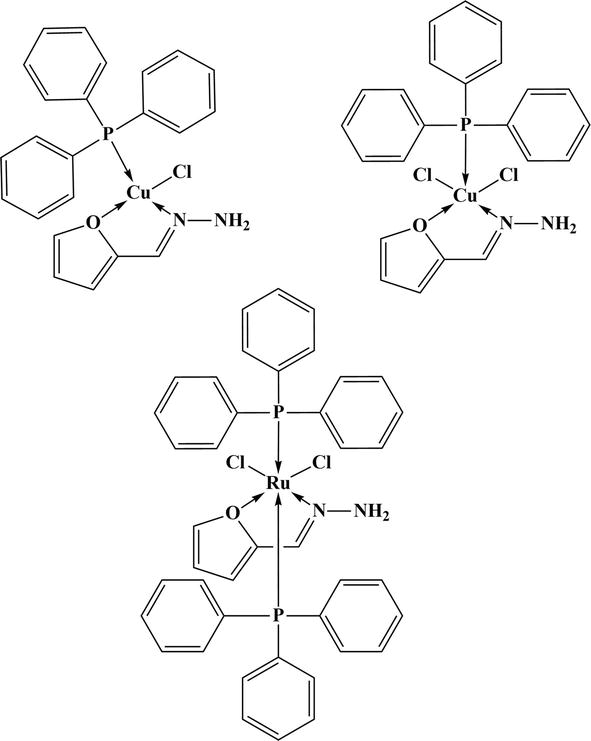
Figure 25.
Metal complexes for the catalytic application in Heck and Suzuki reactions.
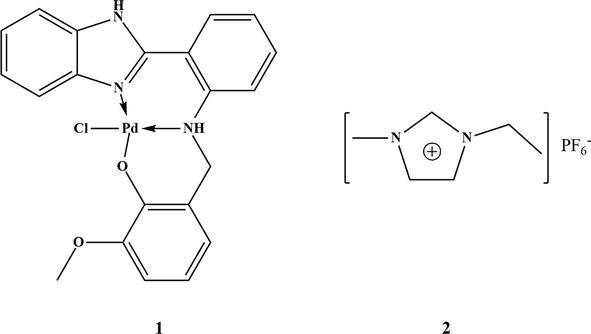
Figure 26.
Structures of benzimidazole-based Schiff base palladium complex (1) and [(EMIM) PF6 (2).
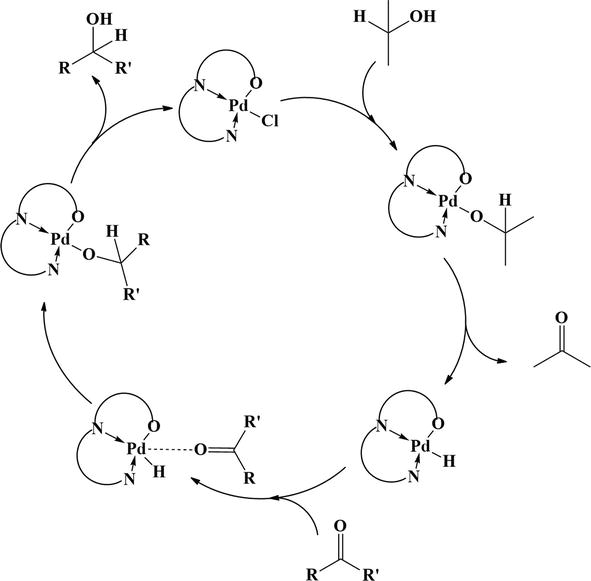
Figure 27.
Mechanism for the palladium complex catalyzed transfer hydrogenation.
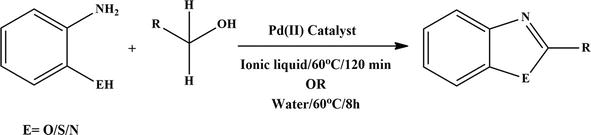
Figure 28.
Synthesis of benzazoles.
6. Conclusions
Schiff’s base properties have been demonstrated to play an important role in many responses as a key to increasing yield and product selection. Important structural kinds and Schiff base ligand types were discovered by the catalytic activity of Schiff base complexes, such as the early oligomerization of isoprene and butadienes. Schiff base and OH group have demonstrated greater olefin epoxidation effectiveness than untreated ligands or aryl. As detailed in this chapter, the flexible metals are combined with Schiff base ligands to assure a good function in changing the reaction to industrial value. Over the years, the structure of the metal structures containing the Schiff base has piqued the public’s interest. Because of their low cost and ease of synthesis, as well as their chemical and thermal stability, Schiff base transition metal complexes are a popular oxidation catalyst for a wide range of organic substrates. Important reactions catalyzed by Schiff base metal complexes include oxidation reactions, reductions, polymerizations, hydroformylation, coupling reactions, the oxidation of sulfides to sulfoxides, alkenes to epoxides and diols, and the activation of hydrocarbons. Yet there is still a huge need for new Schiff bases and their metal complexes as catalysts for organic transformations.
References
- 1.
Yoon TP, Jacobsen EN. Privileged chiral catalysts. Science. 2003; 299 :1691-1693 - 2.
Schiff H. The syntheses and characterization of Schiff base. Annals of Chemistry. 1864; 3 :343-349. DOI: 10.3390/M986 - 3.
Cozzi PG. Metal–Salen Schiff base complexes in catalysis: Practical aspects. Chemical Society Reviews. 2004; 33 (4):410-421 - 4.
Gupta KC, Sutar AK. Catalytic activities of Schiff base transition metal complexes. Coordination Chemistry Reviews. 2008; 252 :1420-1450 - 5.
Arulmurugan S, Helen P, Kavitha VBR. Biological activities of Schiff base and its complexes: A review. Rasayan Journal of Chemistry. 2010; 3 :385-410 - 6.
Huffaker RM. Inhomogeneous radiant heat transfer from Saturn rocket exhaust plumes. No. NASA-TM-X-53630. 1967 - 7.
Yamada S. Advancement in stereochemical aspects of Schiff base metal complexes. Coordination Chemistry Reviews. 1999; 190 :537-555 - 8.
Akhter S, Zaman HU, Mir S, Dar AM, Silaev SS. Synthesis of Schiff base metal complexes: A concise review. European Chemical Bulletin. 2017; 10 :75 - 9.
Akhter S, Zaman HU, Mir S, Dar AM, Silaev SS. Synthesis of Schiff base metal complexes: A concise review. European Chemical Bulletin. 2017; 6 :483 - 10.
Olszwske EJ, Martin DF. Interaction of amines and some nickel (II)-Schiff base compounds-II adduct formation. Journal of Inorganic Nuclear Chemistry. 1965; 27 :345-349 - 11.
Yamanouchi K, Yamada S. Bibliography: General literature. Biological Medicine. 1970; 34 :113-116 - 12.
Cox LR, Ley SV, Diederich F, Stang PJ. Templated organic synthesis. 2000 - 13.
Gimeno N, Vilar R. Anions as templates in coordination and supramolecular chemistry. Coordination Chemistry Reviews. 2006; 250 (23–24):3161-3189 - 14.
Dalia SA, Afsan F, Hossain MS, Khan MN, Zakaria C, Zahan MKE, et al. A short review on chemistry of schiff base metal complexes and their catalytic application. International Journal of Chemical Studies. 2018; 6 :2859-2866 - 15.
Duatti A, Marchi A, Rossi R, Magon L, Deutsch E, Bertolasi V, et al. Reactivity of 2-(2-hydroxyphenyl) benzothiazoline with the oxotetrahalometalate (V) complexes [MOX4]-(M= technetium, rhenium; X= Cl, Br). Synthesis and characterization of new oxo-M (V) complexes containing 2-(2-hydroxyphenyl) benzothiazole. Crystal structure of tetraphenylarsonium oxotrichloro [2-(2-hydroxyphenyl) benzothiazolato] technetate (V). Inorganic Chemistry. 1988; 27 (23):4208-4213 - 16.
Soliman AA, Linert W. Structural features of ONS-donor salicylidene Schiff base complexes. Monatshefte für Chemie-Chemical Monthly. 2007; 138 (3):175-189 - 17.
Kim E, Eduardo E, Chufán KK, Karlin KD. Synthetic models for heme− copper oxidases. Chemical Reviews. 2004; 104 (2):1077-1134 - 18.
Meenachi S, Chitra S. A review of chemistry and biological importance of Schiff base. ChemInform. 2015; 46 :13 - 19.
Manjunatha KB, Supriya S, Shakeel Nawaz S, Ranjan P, Chakraborty T, Poornesh P, et al. Nonlinear optical and quantum chemical studies of palladium benzimidazole Schiff base complex. Materials Science in Semiconductor Processing. 2022; 151 :107012 - 20.
Pawlica D, Marszałek M, Mynarczuk G, Sieroń L, Eilmes J. New unsymmetrical Schiff base Ni (II) complexes as scaffolds for dendritic and amino acid superstructures. New Journal of Chemistry. 2004; 28 (12):1615-1621 - 21.
Costes JP, Dahan F, Fernandez Fernandez MB, Fernandez Garcia MI, Garcia Deibe AM, Sanmartin J. General synthesis of ‘salicylaldehyde half-unit complexes’: Structural determination and use as synthon for the synthesis of dimetallic or trimetallic complexes and of ‘self-assembling ligand complexes’. Inorganica Chimica Acta. 1998; 274 (1):73-81 - 22.
Mukherjee P, Sengupta O, Drew MGB, Ghosh A. Anion directed template synthesis of Cu (II) complexes of a N, N, O donor mono-condensed Schiff base ligand: A molecular scaffold forming highly ordered H-bonded rectangular grids. Inorganica Chimica Acta. 2009; 362 (9):3285-3291 - 23.
Kaczmarek MT, Jastrząb R, Hołderna-Kędzia E, Radecka-Paryzek W. Self-assembled synthesis, characterization and antimicrobial activity of zinc (II) salicylaldimine complexes. Inorganica Chimica Acta. 2009; 362 (9):3127-3133 - 24.
Sumrra SH, Ibrahim M, Ambreen S, Imran M, Rehmani MDFS. Synthesis, Spectral Characterization, and Biological Evaluation of Transition Metal Complexes of Bidentate N, O Donor Schiff Bases. London: Hindawi Publishing Corporation; 2014. pp. 1-10 - 25.
Ambike V, Adsule S, Ahmed F, Wang Z, Afrasiabi Z, Sinn E, et al. Copper conjugates of nimesulide Schiff bases targeting VEGF, COX and Bcl-2 in pancreatic cancer cells. Journal of Inorganic Biochemistry. 2007; 101 (10):1517-1524 - 26.
Patange AN, Yadav UM, Desai PA, Singare PU. Synthesis of some novel halogenated platinum (II) complexes of active Schiff’s base ligand derived from 5-bromo isatin and evaluation of their antibacterial activity. World Scientific News. 2015; 10 :32-43 - 27.
Rosenberg B, Van Camp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965; 205 :698-699 - 28.
Patange AN, Yadav UM, Desai PA, Singare PU. Synthesis and antimicrobial activities of novel palladium (II) complexes of active Schiff’s base ligand derived from 5-bromo isatin. International Letters of Chemistry, Physics and Astronomy. 2015; 52 :22-27 - 29.
Pon S, Mini PS, Theodore David Manickam S, Antony R, Muthupoongodi S, Magala Sathyasheeli S. New class of Copper (II) complex derived from Isatin and Thiosemicarbazide- Synthesis. Der Pharma Chemica. 2016; 8 :67-76 - 30.
Fernández G, Juan M, Fred A, et al. The structures and cyclic voltammetry of three copper (II) complexes derived from bulky ortho-hydroxy Schiff bases. Journal of Molecular Structure. 2002; 612 (1):69-79 - 31.
Baleizao C, Garcia H. Chiral salen complexes: An overview to recoverable and reusable homogeneous and heterogeneous catalysts. Chemical Reviews. 2006; 106 (9):3987-4043 - 32.
Gogoi A, Sarmah G, Dewan A, Bora U. Unique copper–salen complex: An efficient catalyst for N-arylations of anilines and imidazoles at room temperature. Tetrahedron Letters. 2014; 55 (1):31-35 - 33.
Jacobsen EN, Kakiuchi F, Konsler RG, Larrow JF, Tokunaga M. Enantioselective catalytic ring opening of epoxides with carboxylic acids. Tetrahedron Letters. 1997; 38 (5):773-776 - 34.
Gaponik PN, Voitekhovich SV, Ivashkevich OA. Metal derivities of tetrazoles. Russian Chemical Reviews. 2006; 75 (6):507-539 - 35.
Rad MNS. An efficient protocol for facile synthesis of new 5-substituted-1H-tetrazole derivatives using copper-doped silica cuprous sulfate (CDSCS) as heterogeneous nano-catalyst. Journal of the Brazilian Chemical Society. 2017; 28 :11-20 - 36.
Xie A, Cao M, Liu Y, Feng L, Xinyu H, Dong W. The synthesis of tetrazoles in nanometer aqueous micelles at room temperature. European Journal of Organic Chemistry. 2014; 2014 (2):436-441 - 37.
Lauder K, Toscani A, Scalacci N, Castagnolo D. Synthesis and reactivity of propargylamines in organic chemistry. Chemical Reviews. 2017; 117 (24):14091-14200 - 38.
Li P, Regati S, Huang H-C, et al. A sulfonate-based Cu (I) metal-organic framework as a highly efficient and reusable catalyst for the synthesis of propargylamines under solvent-free conditions. Chinese Chemical Letters. 2015; 26 (1):6-10 - 39.
Agrahari B, Layek S, Ganguly R. Synthesis and crystal structures of salen-type Cu (ii) and Ni (ii) Schiff base complexes: Application in [3+ 2]-cycloaddition and a 3-coupling reactions. New Journal of Chemistry. 2018; 42 (16):13754-13762 - 40.
Al-Amin M et al. Selectivity, compatibility, downstream functionalization, and silver effect in the gold and palladium dual-catalytic synthesis of lactones. Organometallics. 2014; 33 (19):5448-5456 - 41.
Li GY. The first phosphine oxide ligand precursors for transition metal catalyzed cross-coupling reactions: C− C, C− N, and C− S bond formation on Unactivated aryl chlorides. Angewandte Chemie International Edition. 2001; 40 (8):1513-1516 - 42.
Suganthy PK, Prabhu RN, Sridevi VS. Synthesis, structure and catalytic applications of octahedral nickel (II) benzoylhydrazone complex: Suzuki–Miyaura cross-coupling reaction of aryl bromides with arylboronic acid. Inorganica Chimica Acta. 2016; 449 :127-132 - 43.
Bazzan I, Volpe A, Dolbecq A, Natali M, Sartorel A, Mialane P, et al. Cobalt based water oxidation catalysis with photogenerated Ru (bpy) 33+: Different kinetics and competent species starting from a molecular polyoxometalate and metal oxide nanoparticles capped with a bisphosphonate alendronate pendant. Catalysis Today. 2017; 290 :39-50 - 44.
Ansari RM, Mahesh LK, Bhat BR. Cobalt Schiff base complexes: Synthesis characterization and catalytic application in Suzuki–Miyaura reaction. 2019; 2019 :556-563 - 45.
Abdel-Fatah SM, Díaz-Sánchez M, Díaz-García D, Prashar S, Abdel-Rahman LH, Gómez-Ruiz S. Nanostructured metal oxides prepared from schiff base metal complexes: Study of the catalytic activity in selective oxidation and C–C coupling reactions. Journal of Inorganic and Organometallic Polymers and Materials. 2020; 30 (4):1293-1305 - 46.
Buldurun K, Özdemir M. Ruthenium (II) complexes with pyridine-based Schiff base ligands: Synthesis, structural characterization and catalytic hydrogenation of ketones. Journal of Molecular Structure. 2012; 2020 :127266 - 47.
Fuchs M, Schmitz S, Schäfer PM, Secker T, Metz A, Ksiazkiewicz AN, et al. Mononuclear zinc (II) Schiff base complexes as catalysts for the ring-opening polymerization of lactide. European Polymer Journal. 2020; 122 :109302 - 48.
Niakan M, Karimi S, Masteri-Farahani M, Shekaari H. An efficient, cost-effective, and magnetically recoverable copper catalyst for O-arylation of phenols with aryl halides in choline chloride-based deep eutectic solvents. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2021; 620 :126603 - 49.
Bunno Y, Tsukimawashi Y, Kojima M, Yoshino T, Matsunaga S. Metal-containing Schiff Base/Sulfoxide Ligands for Pd (II)-Catalyzed Asymmetric Allylic C–H Aminations. ACS Catalysis. 2021; 11 (5):2663-2668 - 50.
Kumar A, Verma S. Synthesis and characterization of a recyclable graphene oxide-surface-engineered copper (II) Schiff base complex: Catalytic application in synthesis of 1, 2, 3-triazoles and 2H-indazoles. Journal of Environmental Chemical Engineering. 2021; 9 (4):105791 - 51.
Ramakrishna D, Bhat BR. Cobalt complexes in [EMIM] Cl–A catalyst for oxidation of alcohols to carbonyls. Inorganic Chemistry Communications. 2010; 13 (1):195 - 52.
Ramakrishna D, Bhat BR. Catalytic oxidation of alcohols by nickel (II) Schiff base complexes containing triphenylphosphine in ionic liquid: An attempt towards green oxidation process. Catalysis Communications. 2010; 11 (5):498 - 53.
Dileep R, Ramachandra Bhat B. Palladium-Schiff base-triphenylphosphine catalyzed oxidation of alcohols. Applied Organometallic Chemistry. 2010; 9 (24):663-666 - 54.
Ramakrishna D. Green conversion of alcohols to carbonyls catalyzed by novel ruthenium-Schiff base-triphenylphosphine complexes. Inorganic Chemistry Communications. 2011; 14 (1):155 - 55.
Ramakrishna D, Bhat BR. A catalytic process for the selective oxidation of alcohols by copper (II) complexes. Inorganic Chemistry Communications. 2011; 14 (5):690 - 56.
Dileep R. Palladium complex in a room temperature ionic liquid: A convenient recyclable reagent for catalytic oxidation. Green Chemistry Letters and Reviews. 2014; 7 (1):32 - 57.
Dileep R, Rudresha BJ. An ionic liquid immobilized copper complex for catalytic epoxidation. RSC Advances. 2015; 5 (81):65870-65873 - 58.
Ramakrishna D. An ionic liquid immobilized palladium complex for an effective heck and Suzuki coupling reactions. Chemistry Africa. 2019; 2 (1):21-28 - 59.
Ramakrishna D. Ionic liquid immobilized Cu (I)–hydrazone–triphenylphosphine complex: An easily recyclable catalytic system for Suzuki and Heck cross couplings. Canadian Journal of Chemistry. 2021; 99 (6):497-501 - 60.
Ramakrishna D, Saravu S, Rajendrachari S. A palladium complex dispersed in ionic liquid as an efficient catalytic combination for the synthesis of Benzazoles. Topics in Catalysis. 2022; 2022 :1-11