Recently synthesized Schiff-base metal complex.
Abstract
Schiff bases are the condensation products of primary amines and carbonyl compounds, which are becoming more and more significant. Schiff bases are imine or azomethine (–C=N–) functional group containing compounds that are produced through a nucleophile addition process. Excellent chelators called Schiff bases have a place in both qualitative and quantitative analysis of metals in aqueous media. Schiff bases were discovered to be auxiliary scaffolds and adaptable pharmacophore for the creation and production of numerous bioactive leads compounds, and this special quality made them accessible for a wide range of biological applications. Schiff bases exhibit significant biological properties including analgesic, anti-inflammatory, antibacterial, anticonvulsant, anti-tubercular, anticancer, antioxidant, anthelmintic antiglycation, and antidepressant activities. In situ cross-linked hydrogel systems are created using the Schiff bases, which are frequently utilized in coordination, organometallic chemistry, and tissue engineering applications. The role of Schiff bases to the design and creation of new lead with potential biological functions is highlighted in this chapter. Researchers’ interest in obtaining the most conclusive and suggestive information on the numerous Schiff bases that have been important for therapeutic purposes over the last few decades and their use in coordination complexes has been maintained by this bioactive core.
Keywords
- Schiff bases
- azomethine
- pharmacophore
- coordination complexes
- biological
1. Introduction
Schiff bases derivatives are novel approaches to researchers for designing heterocyclic/aryl compounds for emergence of new-fangled nature-friendly technology [1]. Schiff bases have been employed as synthons in the generation of various industrial and biological active compounds such as formazans, 4-thiazolidines, benzoxazines, and so on, namely ring closure, cycloaddition, and replacement reactions [2]. It has played an important role in the development of coordination chemistry and was included as key point in the progression of inorganic biochemistry and optical materials [3].
Basically, Schiff bases are the novel approach compounds that possess imine or azomethine (–C=N–) functional group. Hugo Schiff et al. had first reported that Schiff bases are the condensed product of primary amines and carbonyl compounds [4, 5, 6, 7]. In coordination chemistry, Schiff bases are most important class of the widely used organic compounds and have widely application in several fields including inorganic, biological, and analytical chemistry. Schiff bases are important in the therapeutic purposes in medicinal and pharmaceutical field due to its broadly biological activity such as analgesic [8, 9, 10], anti-inflammatory [11], anticancer [12, 13], antioxidant [14], anthelmintic [15], antimicrobial [16, 17], anticonvulsant [18], antitubercular [19], and so on. The presence of N atom of azomethine that involved to form of H-bond with active residue of the protein in the cell and influences cellular mechanisms [20, 21]. Moreover, Schiff bases also play an intermediately role in synthesis of organic compounds, pigments, dyes, polymer stabilizers [7], and for corrosion inhibitors [22]. However, studies revealed that metal complexes of Schiff bases depict more biological activity than free organic compounds [23]. Abdel-Rahman et al. [24] have testified numerous transition metal complexes of Schiff bases as ligands that must be different biological activities, likewise antifungal, anticancer, antibacterial, and so on. As example, Fe (II) complexes have been designed, developed, and synthesized using different Schiff bases ligands that derived from 5- bromosalicylaldehyde and variety of alpha-amino acids such as L-arginine, L-histidine, L-phenylalanine, L-aspartic acid, and L-alanine. Although, these complexes are tested for their antimicrobial activity against various bacterial species such as Escherichia coli, Pseudomonas aeruginosa, and Bacillus cereus. Therefore, it is found that Fe (II) complexes unveiled strong antibacterial activities as compared with amino acid Schiff bases ligands. Moreover, Fe (II) complexes were well interacted with calf thymus DNA using UV-visible spectroscopy, and agarose gel electrophoresis measurement at pH = 7.2. As a result, these complexes showed constant binding with different DNA [25]. However, complexes of Schiff base ligand 2-[(2-Hydroxy-3-methoxy-benzylidene)-amino]pyridin- 3-ol obtained from 2-amino-3-hydroxy-pyridine and 3- methoxysalicylaldehyde with nanosized Fe(II), Cd(II), and Zn(II) metals have been synthesized by sonication method, and all complexes examined for antimicrobial activity against various bacterial species [26]. Recent study revealed that some metal complexes of Schiff bases have greater cytotoxic activity against colon cancerous cell (HCT-116 cell line). Since the middle of the nineteenth century, metal Schiff base complexes have been recognized. Their usage as Schiff base ligands, which are typically monodentate, bidentate, tridentate, tetradentate, etc., and depend on the presence of donor atoms, has been noted in a significant amount of literature. Because they can create stable compounds with transition metals, they are widely used in coordination chemistry [27].
Researchers have focused their research on the chemistry of metal Schiff base complexes with nitrogen and other donor atoms because of their numerous applications in dyes, polymers, enzyme preparation, as well as used as catalyst in various biological systems. This is due to the stability of Schiff base metal complexes as well as biological activity [28], electrochemistry [29], and potential applications in oxidation catalysis [30]. Due to their selectivity, sensitivity, and synthetic flexibility to the central metal atom and the presence of an azomethine group, which aids in elucidating the biological transformation reaction’s mechanism, Schiff bases are widely investigated [31].
Some Cu (II), Co (II), and Ni (II) complexes have been described employing Schiff base ligands generated from 2-amino-3-hydroxypyridine and 3-methoxysalicylaldehyde, and their in vitro antibacterial properties against many bacteria and fungi have been examined. These complexes were further examined for DNA binding, and it was discovered that they could attach to DNA in an intercalative way. However, when compared with the clinically used vinblastine standard, the cytotoxicity of these metal Schiff base complexes on different cell lines such as human colon carcinoma cells (HCT-116 cell line) and breast carcinoma cells (MCF-7 cell line) demonstrated effective cytotoxicity against the growth of carcinoma cells [32]. Che et al. [33] stated that complexes of Pt (II) with Schiff base ligands N,N0-bis(salicylidene)-1,2-ethylenediamine (L1), N,N0- bis(salicylidene)-1,3-propanediamine (L2), and N,N0-bis(salicylidene)- 1,1,2,2-tetramethylethylenediamine (L3) revealed the use of vapor-deposited Pt (II)-salen (11) triplet emitters as for efficient electrophosphorescent dyes in multilayer organic light-emitting diode (OLED) devices with a maximum luminous efficiency of 31 Cd A−1. They discovered that the performance of OLEDs utilizing the Schiff base dopant L3 outperforms that of previously reported Pt (II) emitters.
The majority of Schiff bases are created by condensing salicylaldehyde with both aliphatic and aromatic amines. By using the condensation process of salicylaldehyde with substituted anilines and other aromatic amines, Calvin and Bailes [34] described a number of imines.
These compounds’ intriguing electronic characteristics were discovered through spectroscopic research. According to reports, the existence of a lone pair of electrons in these compounds may explain why there is a stronger ligation with metal ions. In the most recent years, several such azomethines and their complexes with various transition metals have been recorded in review papers [35, 36].
Similarly, Gao and Zheung [37] synthesized Schiff base ligands by condensation of 2-hydroxyacetophenone with various chiral diamines such as 1,2-diamino-cyclohexane, 1,2-diphenylethylenediamine, and 2,20-diamino- 1,10-binaphthalene, respectively, to investigate the steric, electronic, and geometric effect of a methyl (-CH3) group on an azomethine carbon in asymmetric catalytic reaction. Because of the impact of p-conjugation in such luminous complexes, More et al. [37] identified Ni (II) and Zn (II) salophen complexes as potential nonlinear optical materials. A considerable number of Schiff base metal complexes have been shown to be relatively good biological compound models [38].
Schiff bases have played an important part in the advancement of contemporary coordination chemistry, as well as in the advancement of inorganic biochemistry, catalysis, and valuable materials due to optical and magnetic characteristics [39]. In recent years, light emission or charge transport capacity technology has piqued the interest of electronic devices such as solar cells and active components for image and data treatment storage [40]. Metal complexes of Schiff bases contain distinct metals (paramagnetic) groups ascribed to magnetism [41].
2. Synthetic approach for Schiff base
Any primary amine can react with an aldehyde or a ketone under appropriate circumstances to produce Schiff bases. Hugo Schiff (1834–1915), a great chemist who was honored by having Schiff base named in his honor, was born in Frankfurt/Main, Germany’s thriving Jewish community. The Schiff base generally has the following common structure (Figure 1). A Schiff base is a nitrogen analog of an aldehyde or ketone in which the carbonyl group (CO) has been replaced by an imine or azomethine group. It is also known as an imine or an azomethine [42].
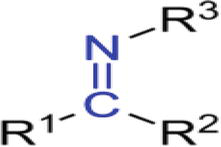
Figure 1.
General structure of Schiff Base (R1 and R2 both would be hydrogens. Alkyl or aryl members made up R3.) [
The library of organic chemistry has a number of amines and carbonyl compounds that make it possible to create Schiff bases with a variety of structural characteristics. The basic carbonyl group for the production of Schiff bases can be an aldehyde (aromatic or aliphatic) or a ketone [43, 44]. The presence of substituent groups connected to the (>C=N) linkage regulates the stability of the imine group. The general reaction for the synthesis of the Schiff base is shown in (Figure 2) where R denotes an alkyl, aryl, cycloalkyl, or heterocyclic group, which might be variably substituted, and R1 may be an alkyl, aryl group, or H atom [46]. Refluxing the mixture under neutral conditions or with acid or basic catalysts usually causes the Schiff base to develop, which is a reversible process. Usually, the product separation or water removal is what brings the formation to completion [47].
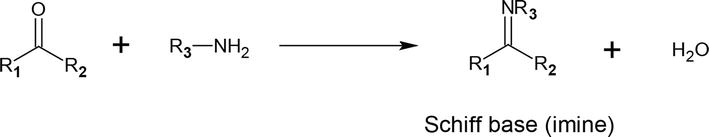
Figure 2.
Schiff base formation reaction scheme [
Schiff bases are still synthesized by chemists, and today, active and well-designed Schiff base ligands are referred to as “privileged ligands.” The bridging Schiff’s bases have the following structure, which has a variety of functional groups that can be altered to suit different needs. R” is phenyl or a substituted phenyl, H is an alkyl or aryl group, and X is a phenyl or substituted phenyl group. In fact, Schiff bases have the ability to stable a wide range of metals in a variety of oxidation states, regulating the performance of metals in a wide range of advantageous catalytic transformations. The oxygen atoms in Schiff bases can be changed to sulfur, nitrogen, or selenium atoms; however, NO or N2O2-donor groups are the most frequent donor groups [48].
The mechanism of forming a Schiff base (Figure 3) is an additional application of the nucleophilic addition to the carbonyl group. The amine is the nucleophile in this situation. The amine interacts with the aldehyde or ketone in the first step of the process to produce carbinolamine, an unstable addition product. By bases or acids catalyzing the process, the carbinolamine loses water. The dehydration of the carbinolamine is acid-catalyzed because it is an alcohol. The reaction is typically catalyzed by acids because the dehydration of the carbinolamine is the rate-determining step in the production of the Schiff base. However, because amines are basic molecules, the acid concentration cannot be too high. The equilibrium is pulled to the left, and carbinolamine production is prevented if the amine is protonated and turns non-nucleophilic. As a result, low acidity is preferred for many Schiff base synthess. Base can also catalyze the dehydration of carbinolamines. Despite not being a concerted reaction, this reaction is somewhat comparable to the E2 elimination of alkyl halides. Through an anionic intermediate, it moves forward in two steps. The process of creating a Schiff base actually involves two different types of reactions, addition and elimination [48, 49].
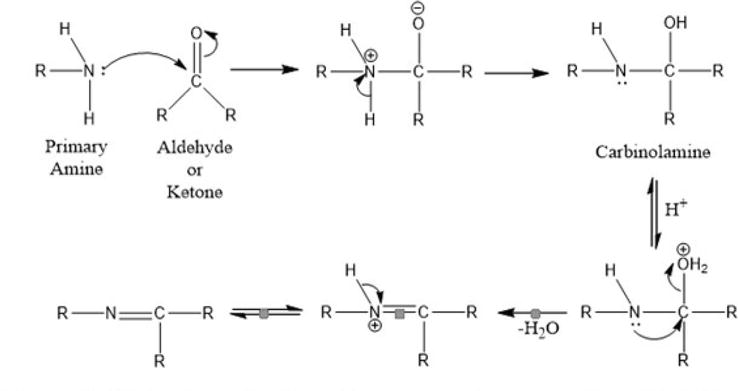
Figure 3.
Mechanism of Schiff base formation [
In the meantime, a number of methods and systems for the synthesis of Schiff base have been described, including NaHCO3 [44], CuSO4 [46], P2O5/Al2O3 [47], MgClO4 [45], ZnCl2 [50], and MgSO4-PPTS [51]. In these systems, metal species act as Lewis acids to activate the carbonyl group and facilitate the removal of water. A few developments have been reported in recent years due to the advancement of experimental procedures, such as solid-state synthesis [52], solvent-free/clay/microwave irradiation [53], water suspension medium [54], reflux/solvent [55], infrared irradiation/no solvent [56], and K-10/microwave. The mentioned methods/systems revealed some drawbacks, including the need for high reaction temperatures, prolonged reaction times, and moisture-sensitive catalysts, huge amounts of aromatic solvents, expensive dehydrating reagents/catalysts, and specialized equipment [57]. NaHSO4.SiO2/microwave/solvent-free [58], dirhodium caprolactamate [59], [bmim] BF4/molecular sieves [60], silica/ultrasound irradiation [61], silica/microwave [19], and silica/solvent-free [62].
3. Spectroscopic analysis of Schiff base
A Schiff base is a compound with the general structure R1R2C=NR’. They can be considered a subclass of imines, which is also synonymous with azomethine [63]. In order to investigate hybrid composites, spectroscopic analysis is used. The analysis reveals useful details such as elemental type, chemical composition, optical and electronic properties, and crystallinity. Ultraviolet and visible light (UV-vis) spectroscopy, elemental analysis, differential scanning calorimetric (DSC), hydrogen nuclear magnetic resonance (1H NMR), and Fourier transform infrared (FTIR) studies are used to evaluate these Schiff bases [64].
In the IR spectra, C=N is most commonly reported in the 1690–1640 cm−1 region as a strong and a sharp band at somewhat lower frequencies than the bands of C=O groups and close to C=C stretching frequencies. With angle strain, steric repulsion, other complicated local factors, solution concentration and nature of solvent, the stretching frequency of C=N is found to be at 1670 cm−1. The frequency is usually lowered in the absence of one or more groups in conjugation with the C=N [65]. The multinuclear (1H and 13C) NMR spectral analyses are helpful to characterize and confirm the structure of Schiff bases. The upfield and downfield shifting of the signal is dependent upon the substituents present over the Schiff bases. In the CHN analysis of the Schiff bases, the elemental and sometimes isotopic compositions were found out for the confirmation of the structure of the synthesized derivatives.
It is important to note that the nitrogen atom in the Schiff base has a lone pair, which gives it the characteristics of a Lewis base and allows it to participate in the creation of hydrogen bonds, either intramolecularly or with polar molecules. This characteristic encourages the development of intramolecular hydrogen bonding, particularly when suitable non-polar solvents are present [66].
4. Schiff-base metal complexes
Schiff-base metal complexes have been considered the active topic of research in coordination chemistry during a few decades of extensive research on metal-based pharmaceuticals, owing to their useful applications in numerous disciplines of science. They have potential therapeutic applications as antibiotics, antimicrobials, antitumors, antivirals, anti-inflammatory medicines, analgesics, antifungals, and many others [47]. Schiff bases are versatile pharmacophores that trap in metal ions within their structural units due to the presence of multiple donor atoms [67]. Schiff bases (azomethines) are formed by combining amino and carbonyl groups with multidentate ligands and forming highly significant complexes with metal ions. By using azomethine nitrogen, they can coordinate with metal ions. In organic synthesis, the Schiff base reaction is fundamental for the synthesis of C-N bonds. (Figure 4) They exhibit chelation property with O, N, and S donors, and metal complexes have a diverse biological action against many infections and cancers. Schiff base complexes with multidentate ligands are capable of chelating any metal ions. These ligands are effective in the exciting unique therapeutic approach to better understanding diseases and their therapy. The complexes of Schiff base of both transition metal ions (i.e., inner and outer) containing NO or NOS donor atoms were described as playing a significant role in biological activities. Because they are colorful and very stable for biological activities, certain of these metal complexes have attractive physical and chemical properties [69]. Some of the recently synthesized Schiff-base metal complexes are enlisted in Table 1.
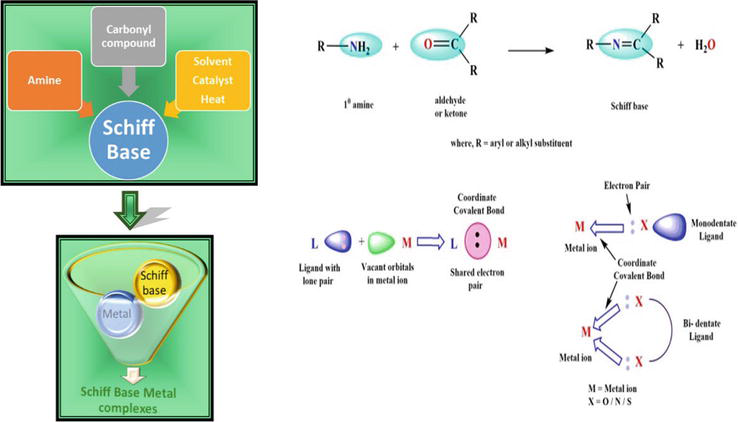
Figure 4.
Formation of Schiff-base metal complexes [
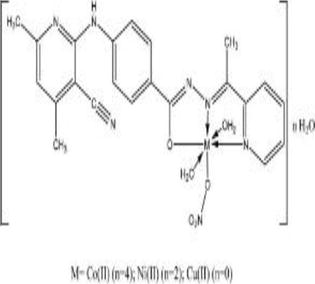
Table 1.
5. Schiff base biological activity
Bacteria that display multiple antibiotic resistance [77] are closely linked to the rise in the mortality rate of infectious illnesses [78]. The main reason for this issue is the lack of efficient therapies. There is unquestionably an urgent medical need for the creation of new antibacterial drugs with inventive and more effective modes of action. Hence, Schiff bases called azomethines containing nitrogenous analogue of carbonyl compound changed by imine group are used. For antimicrobial activity, these bases produce excellent activity [79]. Aside from natural and synthetic molecules, biomolecules, sulfonamides, coumarins, aminothiazolyl, bromocoumarins, O-phthaldehyde, or 2-aminophenol, and 1, 2, 4-triazoles are other molecules that can be used as platforms to create Schiff bases for antibacterial activities [80].
As malaria is a disorder associated with severe health problems of public and globally its cases are increasing. Hence, various authors synthesized antimalarial Schiff base derivatives for prominent activity [81]. Also these bases have maximum activity for fungal infection due to its structure. In case of viral disorder, in the market there are numbers of drugs, but they are not fully active due to mutations in viruses. To overcome these problems, Schiff base derivatives are synthesized as an antiviral agent [82]. Different biological activities of Schiff bases are shown in Table 2.
Table 2.
Different biological activities of Schiff bases.
6. Schiffs base abiological activity
Schiff base possesses various medicinal activities. In addition to the same, they are now being used in diagnostics also. Some of the abioloical applications of Schiff’s base are mentioned in this section.
6.1 Bioimaging applications
A variety of Schiff base probes with fluorescent sensors are used for bioimaging applications to detect metal ions. Most of the fluorescent probes selectively and sensitively detect only one or two metal ions. Schiff base probes detect analytes in non-aqueous or semi-aqueous media, making them useful for the detection and monitoring of toxic ions in drinking water and industrial waste [100]. A simple and versatile Schiff base chemical sensor was developed to detect four adjacent series 4 metal ions (Co2+, Ni2+, Cu2+, and Zn2+) by colorimetric or fluorometric methods. This chemical sensor has been used to image Zn2+ in HepG2 cells, zebrafish, and tumor-bearing mice, demonstrating potential biological applications [101]. The fluorescence sensor synthesized from the reaction of picolinohydrazide and 4(diethylamino) salicylaldehyde successfully detected Al3+ and Zn2+ in living cells, suggesting that this simple biosensor has great potential for biological imaging applications [102].
Compound (E)-1-((L-glutaminoimino) methyl)naphthalene-2-ol (A) showed good solubility and compatibility in the presence or absence of Al3+ and showed some fluorescence in human Hs27 epithelial cells. Bioimaging has been reported [4]. Fluorescent Schiff base organotin dyes (1: Et2N-L-SnPh2, 2: Et2N-L-SnBu2, 3: MeO-L-SnPh2, 4: MeO-L-SnBu2, 5: HO-L-SnPh2, and 6: HO − L-SnBu2, L = 2-hydroxybenzylidene-4-hydroxybenzhydrazine) showed efficient two-photon excitation (1–4). Two of the compounds (5 and 6) were found to be able to selectively accumulate in HeLa cells, allowing their differentiation from normal cells (periodontal ligament cells) [103, 104].
6.2 Phosphorescent OLEDS
Five one-armed Schiff base ligands HL1, HL2, HL3, HL4, and HL5 were obtained from condensation of various group-substituted salicylaldehydes with aniline and 2,4,5-trifluoroaniline gave. Their platinum(II) complexes Pt(L1)2, Pt(L2)2, Pt(L3)2, Pt(L4)2, Pt(L5)2, and PtL5 DMSO obtained by the metalation of ligands with K2PtCl4 were found to be excellent candidates for phosphorescent OLEDs [105]. The luminescent performance of azomethine zinc complexes in organic light-emitting diodes has been investigated, and the results have shown excellent electroluminescence properties as blue fluorescent light sources [106]. Schiff-base zinc metal complexes have been developed to serve as efficient light-emitting materials for optoelectronic applications such as organic light-emitting diodes. These zinc complexes serve as promising emissive layers for optoelectronic applications [107].
6.3 Sensing applications
Fluorescence on/off sensor of a wide range of Schiff bases is being developed for determination of various analytes, toxic ions, and metallic cations and anions in different types of environmental and biological media [108].
Biosensor: Conductive hydrogels based on graphene oxide, dopamine, and polyacrylamide were prepared using the Schiff bases. The high elongation, toughness, and self-adhesion of conducting hydrogels have provided great advantages as biosensors [109].
In forensics, Schiff bases are primarily used in the analysis of illicit drugs. Chemical reactions with Schiff bases reveal illicit drug production and help determine analytes in confiscated samples [110, 111].
6.4 Tissue regeneration
Various substituted Schiff bases have enhanced bulk modulus of the composite hydrogels and slightly increased the in vitro degradation rate. It also promoted cell adhesion and proliferation and maintains the regular cell morphology of bovine articular chondrocytes, increasing potential applications in cartilage tissue engineering [109].
6.5 Bioprint
Water-soluble hydroxybutyl chitosan (HBC) and chondroitin oxysulfate (OCS) have been used to generate bioinks based on the Schiff base reaction, using different sacrificial molds in 3D bioprinting techniques to produce different structures of hydrogels. The controllable shape of HBC/OCS bionic hydrogels can be optimized and customized for specific cartilage engineering applications [109].
6.6 Tissue adhesion
Aldehyde groups in hydrogels based on the Schiff reagent can promote the adhesion of hydrogels to surrounding tissues. An injectable double-cross-linked self-healing hydrogel based on dopamine-grafted oxidized sodium alginate (OSA-DA) and polyacrylamide (PAM) for wound healing has been reported. In terms of hydrogen bonding and Schiff base bonding, the self-healing OSA-DA-PAM hydrogel possesses stable mechanical properties such as high tensile strength and elongation. In addition, numerous catechol groups on OSA-DA chains can endow hydrogels with unique cell affinity and tissue adhesion [10]. In situ forming hydrogel, derived from natural polysaccharides through Schiff base reaction, can be modulated and prepared for soft tissue adhesive, hemostasis, or other biomedical applications in future [110].
6.7 Dyes
New complexes of Zn(II), Pd(II), and Pt(II) with Schiff bases are metal salts of 4-(dibutylamino)-2-hydroxybenzaldehyde and 4,5-diaminophthalonitrile. Sensor applications for imaging surface temperature (planar optodes) and monitoring rapid temperature changes (fiber optic microsensors) have been demonstrated. Pt(II) complexes immobilized in gas permeable matrices also turned out to be promising materials for oxygen measurements. [112].
Schiff bases based on salicylaldehyde units and their use as metal-free organic chromophores can be used to sensitized and co-sensitize dye-sensitized solar cells (DSSCs) [113].
Compounds Et2N-L-SnPh2 and MeO-L-SnPh2 act as an excellent staining for cancer cells (HeLa) using two-photon bioimaging and are expected to have biomedical applications [104].
7. Future prospectus
From the discussion in the section of biological evaluation of Schiff bases, it was clear that the pharmacophore possesses various biological activities, which can be again explored more against various diseases. New groups of organic compounds are still being described, the combinations of which may form a group of extremely desirable compounds with higher potential. The biologically active Schiff’s base ligands and metal complexes are playing very crucial role in the drug discovery [114].Medicinal chemists are now interested in developing novel chemotherapeutic Schiff bases and their metal complexes.
Apart from this, conjugated Schiff Bases have been employed in electronics such the organic field-effect transistor, Perovskite solar cells, and electrochromic devices because they offer some intriguing optoelectronic features. These are also employed in the production of covalent organic framework, which is used in the storage of gas [115]. The measurement of pH values has evolved into one of the most essential necessities with the recent advancements in biological and environmental research. Because of their smooth synthetic roots, easily tuneable structural architecture, nondestructive signals of emission, visually differentiable color generation, and capacity for real sample analysis, organic Schiff base compounds and their derivatives have been observed to play crucial roles in determining the pH values of a particular medium [116]. Because of its bioactive core, Schiff bases have a wide range of applications in the chemical, food, coordination, medical, agricultural, and other industries. This body of literature made it very evident that there will be several opportunities for Schiff bases to become active research molecules in the future.
8. Conclusion
Schiff bases are integral core of the organic chemistry having a verity of biological activities. This chapter focused on the novel leads of numerous Schiff bases having potential medicinal activities with lesser side effects. At last decades, the researchers perceived attention toward bioactive core of Schiff bases, which gained wide medicinal interest. This chapter also explored industrial application of Schiff bases. Advances in this era will require access of the structure activity relationship and mechanism of action of the Schiff bases containing compounds.
At present scenario, Schiff bases are perceived importance in biological activity. It is a versatile organic compound, which is synthesized in reaction between amino compound and aldehyde or ketone well known as imine by condensation process. This imine or azomethine functional groups are versatile pharmacophores for design and development of various bioactive lead compounds. Schiff bases are extensively used for biological activities such antimicrobial, anthelmintic, anti-inflammatory, anticonvulsant, antiTB, antineoplastic, and antidepressant activities. Also, Schiff bases have an intermediately compound in synthesis of various organic compounds. It is also used as catalyst, pigments, and dye synthesis. Furthermore, Schiff bases are used as corrosion inhibitors. The metal complexes of Schiff bases have diversified biological activities. The present chapter summarized the information with respect to diverse biological activities and depicted the recent synthesized variety Schiff bases as potential bioactive core.
References
- 1.
Bhattacharya A, Purohit VC, Rinaldi F. Environmentally friendly solvent-free processes: Novel dual catalyst system in Henry reaction. Organic Process Research and Development. 2003; 7 (3):254-258 - 2.
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel JM. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules. 2007; 12 (8):1720-1730 - 3.
Tisato F, Refosco F, Bandoli G. Structural survey of technetium complexes. Coordination Chemistry Reviews. 1994; 135–136 :325-397 - 4.
Cimerman Z, Miljanic S, Galic N. Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croatica Chemica Acta. 2000; 73 (1):81-95 - 5.
Schiff H. Mittheilungen aus dem Universit¨atslaboratorium in Pisa: eine neue reihe organischer Basen. Justus Liebigs Annalen der Chemie. 1864; 131 (1):118-119 - 6.
Dhar DN, Taploo CL. Schiff bases and their applications. Journal of Scientific and Industrial Research. 1982; 41 (8):501-506 - 7.
Sathe BS, Jaychandran E, Jagtap VA, Sreenivasa GM. Synthesis characterization and anti-inflammatory evaluation of new fluorobenzothiazole schiff’s bases. International Journal of Pharmaceutical Research and Development. 2011; 3 (3):164-169 - 8.
Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/ benzoxazole derivatives and some Schiff’s bases. Bioorganic and Medicinal Chemistry. 2006; 14 (11):3758-3765 - 9.
Pandey A, Dewangan D, Verma S, Mishra A, Dubey RD. Synthesis of schiff bases of 2-amino-5-aryl-1,3,4-thiadiazole and its analgesic, anti-inflammatory, anti-bacterial and antitubercular activity. International Journal of ChemTech Research. 2011; 3 (1):178-184 - 10.
Chandramouli C, Shivanand MR, Nayanbhai TB, Bheemachari B, Udupi RH. Synthesis and biological screening of certain new triazole schiff bases and their derivatives bearing substituted benzothiazole moiety. Journal of Chemical and Pharmaceutical Research. 2012; 4 (2):1151-1159 - 11.
Chinnasamy RP, Sundararajan R, Govindaraj S. Synthesis, characterization, and analgesic activity of novel Schiff base of isatin derivatives. Journal of Advanced Pharmaceutical Technology and Research. 2010; 1 (3):342-347 - 12.
Venkatesh P. Synthesis, characterization and antimicrobial activity of various schiff bases complexes of Zn(II) and Cu(II) ions. Asian Journal of Pharmaceutical and Health Sciences. 2011; 1 (1):8-11 - 13.
Chaubey AK, Pandeya SN. Synthesis & anticonvulsant activity (chemo shock) of Schiff and Mannich bases of Isatin derivatives with 2-amino pyridine (mechanism of action). International Journal of PharmTech Research. 2012; 4 (4):590-598 - 14.
Aboul-Fadl T, Mohammed FA, Hassan EA. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1- alkylisatin and isonicotinic acid hydrazide (INH). Archives of Pharmacal Research. 2003; 26 (10):778-784 - 15.
Miri R, Razzaghi-asl N, Mohammadi MK. QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. Journal of Molecular Modeling. 2013; 19 (2):727-735 - 16.
Mounika K, Anupama B, Pragathi J, Gyanakumari C. Synthesis, characterization and biological activity of a Schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. Journal of Scientific Research. 2010; 2 (3):513-524 - 17.
Ali SMM, Azad MAK, Jesmin M, et al. In vivo anticancer activity of vanillin semicarbazone. Asian Pacific Journal of Tropical Biomedicine. 2012; 2 (6):438-442 - 18.
Wei D, Li N, Lu G, Yao K. Synthesis, catalytic and biological activity of novel dinuclear copper complexwith Schiff base. Science in China B. 2006; 49 (3):225-229 - 19.
Avaji PG, Vinod Kumar CH, Patil SA, Shivananda KN, Nagaraju C. Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. European Journal of Medicinal Chemistry. 2009; 44 (9):3552-3559 - 20.
Venugopala KN, Jayashree BS. Synthesis of carboxamides of 2-amino-4-(6-bromo-3-coumarinyl) thiazole as analgesic and antiinflammatory agents. Indian Journal of Heterocyclic Chemistry. 2003; 12 (4):307-310 - 21.
Vashi K, Naik HB. Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. European Journal of Chemistry. 2004; 1 :272-276 - 22.
Li S, Chen S, Lei S, Ma H, Yu R, Liu D. Investigation on some Schiff bases as HCl corrosion inhibitors for copper. Corrosion Science. 1999; 41 (7):1273-1287 - 23.
Chohan ZH, Praveen M, Ghaffar A. Structural and biological behaviour of Co(II), Cu(II) and Ni(II) metal complexes of some amino acid derived Schiff-bases. Metal-Based Drugs. 1997; 4 (5):267-272 - 24.
Abdel-Rahman LH, Abu-Dief AM, El-Khatib RM, Abdel-Fatah SM. Some new nano-sized Fe (II), Cd (II) and Zn (II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorganic Chemistry. 1 Dec 2016; 69 :140-152 - 25.
Malladi S, Isloor AM, Isloor S, Akhila DS. Synthesis, characterization and antibacterial activity of some newpyrazole based Schiff bases. Arabian Journal of Chemistry. 2013; 6 (3):335-340 - 26.
Essa HH, Kandil F, Falah A. Synthesis and identification of schiff bases and biological activity new study. Iraqi Journal of Science. 2012; 53 (2):230-240 - 27.
Shelke VA, Jadhav SM, Patharkar VR, Shankarwar SG, Munde AS, Chondhekar TK. Synthesis, spectroscopic characterization and thermal studies of some rare earth metal complexes of unsymmetrical tetradentate Schiff base ligand. Arabian Journal of Chemistry. 2012; 5 :501-507 - 28.
Sheikh RA, Wani MY, Shreaz S, Hashmi AA. Synthesis, characterization and biological screening of some Schiff base macrocyclic ligand based transition metal complexes as antifungal agents. Arabian Journal of Chemistry. 1 Sep 2016; 9 :S743-S751 - 29.
Sashidhara KV, Kumar A, Bhatia G, Khan MM, Khanna AK, Saxena JK. Antidyslipidemic and antioxidative activities of 8-hydroxyquinoline derived novel keto-enamine Schiffs bases. European Journal of Medicinal Chemistry. 2009; 44 (4):1813-1818 - 30.
Sashidhara KV, Rosaiah JN, Bhatia G, Saxena JK. Novel keto-enamine Schiffs bases from7-hydroxy-4-methyl-2-oxo-2H-benzo[h] chromene-8,10-dicarbaldehyde as potential antidyslipidemic and antioxidant agents. European Journal of Medicinal Chemistry. 2008; 43 (11):2592-2596 - 31.
Revanasiddappa HD, Prasad KS, Kumar LS, Jayalakshmi B. Synthesis and biological activity of new Schiff bases containing 4(3H)-quinazolinone ring system. International Journal of ChemTech Research. 2010; 2 (2):1344-1349 - 32.
Kundu A, Shakil NA, Saxena DB, Pankaj P, Kumar J, Walia S. Microwave assisted solvent-free synthesis and biological activities of novel imines (Schiff bases). Journal of Environmental Science and Health B. 2009; 44 (5):428-434 - 33.
Che CM, Chan SC, Xiang HF, Chan MC, Liu Y, Wang Y. Tetradentate Schiff base platinum (II) complexes as new class of phosphorescent materials for high-efficiency and white-light electroluminescent devices. Chemical Communications. 2004;(13):1484-1485 - 34.
Aboul-Fadl T, Bin-Jubair FAS, Aboul-Wafa O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. European Journal of Medicinal Chemistry. 2010; 45 (10):4578-4586 - 35.
Ferreira MDL, Vasconcelos TRA, de Carvalho EM, et al. Synthesis and antitubercular activity of novel Schiff bases derived from d-mannitol. Carbohydrate Research. 2009; 344 (15):2042-2047 - 36.
Thomas AB, Nanda RK, Kothapalli LP, Hamane SC. Synthesis and biological evaluation of Schiff’s bases and 2-azetidinones of isonocotinyl hydrazone as potential antidepressant and nootropic agents. Arabian Journal of Chemistry. 1 Sep 2016; 9 :S79-S90 - 37.
Gao WT, Zheng Z. Synthetic studies on optically active Schiff-base ligands derived from condensation of 2-hydroxyacetophenone and chiral diamines. Molecules. 31 Jul 2002; 7 (7):511-516 - 38.
More MS, Devkule SS, Chavan SS. Ni (II) and Zn (II) complexes containing alkynyl functionalized salicylaldimine ligand and heterocyclic coligand: Synthesis, characterization and luminescence properties. Journal of Fluorescence. May 2017; 27 (3):841-851 - 39.
Aly MM, Mohamed YA, El-Bayouki KAM, Basyouni WM, Abbas SY. Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities e Part-1. European Journal of Medicinal Chemistry. 2010; 45 (8):3365-3373 - 40.
Nithinchandra B, Kalluraya SA, Shabaraya AR. Regioselective reaction: Synthesis, characterization and pharmacological activity of some new Mannich and Schiff bases containing sydnone. European Journal of Medicinal Chemistry. 2012; 54 :597-604 - 41.
Alam MS, Choi J, Lee D. Synthesis of novel Schiff base analogues of 4-amino-1, 5-dimethyl-2- phenylpyrazol-3- one and their evaluation for antioxidant and anti-inflammatory activity. Bioorganic & Medicinal Chemistry. 2012; 20 :4103-4108 - 42.
Da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CV, et al. Schiff bases: A short review of their antimicrobial activities. Journal of Advanced Research. 2011; 2 (1):1, 1-8 - 43.
Zheng Y, Ma K, Li H, Li J, He J, Sun X, et al. One pot synthesis of imines from aromatic nitro compounds with a novel Ni/SiO2 magnetic catalyst. Catalysis Letters. 2009 Mar; 128 (3):465-474 - 44.
Samec JS, Bäckvall JE. Ruthenium-catalyzed transfer hydrogenation of imines by Propan-2-ol in benzene. Chemistry–a. European Journal. 2002; 8 (13):2955-2961 - 45.
Kumar KS, Bayeh Y, Gebretsadik T, Elemo F, Gebrezgiabher M, Thomas M, et al. Spin-crossover in iron (ii)-Schiff base complexes. Dalton Transactions. 2019; 48 (41):15321-15337 - 46.
Jennings WB, Lovely CJ. An efficient method for the preparation of N-phosphinoyl and N-sulphonyl imines directly from aromatic aldehydes. Tetrahedron Letters. 1988; 29 (30):3725-3728 - 47.
Chaudhary NK, Guragain B, Chaudhary SK, Mishra P. Schiff base metal complex as a potential therapeutic drug in medical science: A critical review. Bibechana. 2021; 18 (1):214-230 - 48.
Anamika S, Chaturvedi P. Review on Schiff bases. World Journal of Pharmaceutical Sciences. 2021; 27 :133-140 - 49.
Naeimi H, Salimi F, Rabiei K. Mild and convenient one pot synthesis of Schiff bases in the presence of P2O5/Al2O3 as new catalyst under solvent-free conditions. Journal of Molecular Catalysis A: Chemical. 2006; 260 (1–2):100-104 - 50.
Billman JH, Tai KM. Reduction of schiff bases. II. Benzhydrylamines and structurally related compounds1a, b. The Journal of Organic Chemistry. 1958; 23 (4):535-539 - 51.
Chakraborti AK, Bhagat S, Rudrawar S. Magnesium perchlorate as an efficient catalyst for the synthesis of imines and phenylhydrazones. Tetrahedron Letters. 2004; 45 (41):7641 - 52.
Schmeyers J, Toda F, Boy J, Kaupp G. Quantitative solid–solid synthesis of azomethines. Journal of the Chemical Society, Perkin Transactions. 1998; 2 (4):989-994 - 53.
Varma RS, Dahiya R, Kumar S. Clay catalyzed synthesis of imines and enamines under solvent-free conditions using microwave irradiation. Tetrahedron Letters. 1997; 38 (12):2039-2042 - 54.
Tanaka K, Shiraishi R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chemistry. 2000; 2 (6):272-273 - 55.
Casey CP, Johnson JB. Isomerization and deuterium scrambling evidence for a change in the rate-limiting step during imine hydrogenation by Shvo's hydroxycyclopentadienyl ruthenium hydride. Journal of the American Chemical Society. 2005; 127 (6):1883-1894 - 56.
Vázquez MÁ, Landa M, Reyes L, Miranda R, Tamariz J, Delgado F. Infrared irradiation: Effective promoter in the formation of n-benzylideneanilines in the absence of solvent. Synthetic communications. 2004; 34 (15):2705-2718 - 57.
Guzen KP, Guarezemini AS, Orfao AT, Cella R, Pereira CM, Stefani HA. Eco-friendly synthesis of imines by ultrasound irradiation. Tetrahedron Letters. 2007; 48 (10):1845-1848 - 58.
Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R. Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. Journal of Chemical Research. 2005; 2005 (5):299-303 - 59.
Choi H, Doyle MP. Oxidation of secondary amines catalyzed by dirhodium caprolactamate. Chemical Communications. 2007; 7 :745-747 - 60.
Andrade CK, Takada SC, Alves LM, Rodrigues JP, Suarez PA, Brandao RF, et al. Molecular sieves in ionic liquids as an efficient and recyclable medium for the synthesis of imines. Synlett. 2004; 2004 (12):2135-2138 - 61.
Venugopala KN, Jayashree BS. Microwave-induced synthesis of Schiff bases of aminothiazolyl bromocoumarins as antibacterials. Indian Journal of Pharmaceutical Sciences. 2008; 70 (1):88 - 62.
Kagatikar S, Sunil D. Schiff bases and their complexes in organic light emitting diode application. Journal of Electronic Materials. 2021; 50 (12):6708-6723 - 63.
Ceramella J, Iacopetta D, Catalano A, Cirillo F, Lappano R, Sinicropi MS. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics. 2022; 11 (2):191 - 64.
Jarząbek B, Kaczmarczyk B, Sęk D. Characteristic and spectroscopic properties of the Schiff-base model compounds. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2009; 74 (4):949-954 - 65.
Rodríguez MR, Del Pla J, Piro OE, Echeverría GA, Espino G, Pis-Diez R, et al. Structure, tautomerism, spectroscopic and DFT study of o-vanillin derived Schiff bases containing thiophene ring. Journal of Molecular Structure. 2018; 1165 :381-390 - 66.
Raczuk E, Dmochowska B, Samaszko-Fiertek J, Madaj J. Different Schiff bases—Structure, importance and classification. Molecules. 2022; 27 :787 - 67.
Kajal A, Bala S, Kamboj S, Sharma N, Saini V. Schiff bases: A versatile pharmacophore. Journal of Catalysts. 2013; 2013 :27 - 68.
Jain A, De S, Barman P. Microwave-assisted synthesis and notable applications of Schiff-base and metal complexes: A comparative study. Research on Chemical Intermediates. 2022; 48 (5):2199-2251 - 69.
Ghanghas P, Choudhary A, Kumar D, Poonia K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorganic Chemistry Communications. 2021; 130 :108710 - 70.
Vijayan T, Kim J, Azam M, Al-Resayes SI, Stalin A, Kannan BS, et al. Influence of co-ligand on the biological properties of Schiff base metal complexes: Synthesis, characterization, cytotoxicity, and antimicrobial studies. Applied Organometallic Chemistry. 2022; 36 (3):e6542 - 71.
El-Gammal OA, El-Bindary AA, Mohamed FS, Rezk GN, El-Bindary MA. Synthesis, characterization, design, molecular docking, anti COVID-19 activity, DFT calculations of novel Schiff base with some transition metal complexes. Journal of Molecular Liquids. 2022; 346 :117850 - 72.
Hashem HE, Nath A, Kumer A. Synthesis, molecular docking, molecular dynamic, quantum calculation, and antibacterial activity of new Schiff base-metal complexes. Journal of Molecular Structure. 2022; 1250 :131915 - 73.
Howsaui HB, Sharfalddin AA, Abdellattif MH, Basaleh AS, Hussien MA. Synthesis, spectroscopic characterization and biological studies of Mn (II), Cu (II), Ni (II), Co (II) and Zn (II) complexes with new Schiff Base of 2-((Pyrazine-2-ylimino) methyl) phenol. Applied Sciences. 2021; 11 (19):9067 - 74.
Naureen B, Miana GA, Shahid K, Asghar M, Tanveer S, Sarwar A. Iron (III) and zinc (II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. Journal of Molecular Structure. 2021; 1231 :129946 - 75.
Shiju C, Arish D, Kumaresan S. Novel water soluble Schiff base metal complexes: Synthesis, characterization, antimicrobial-, DNA cleavage, and anticancer activity. Journal of Molecular Structure. 2020; 1221 :128770 - 76.
Dhanaraj CJ, Raj SS. Synthesis, characterization and biological studies of Schiff base metal complexes derived from 4-aminoantipyrine, acetamide and p-phenylenediamine. Inorganic Chemistry Communications. 2020; 119 :108087 - 77.
Frieri M et al. Antibiotic resistance. Journal of Infectious Public Health. 2016:S18 - 78.
Kimberly W, John D. Global trends in infectious diseases of swine. Proceedings of the National Academy of Sciences. 2017; 2017 :201 - 79.
Cinarli A, Gürbüz D, Tavman A, Birteksöz AS. Synthesis, spectral characterizations and antimicrobial activity of some Schiff bases of 4-chloro-2-aminophenol. Bulletin of the Chemical Society of Ethiopia. 2011; 25 (3):407-417 - 80.
Karrouchi K, Chemlal L, Taoufik J, Cherrah Y, Radi S, El Abbes FM, et al. Synthesis, antioxidant and analgesic activities of Schiff bases of 4-amino-1,2,4-triazole derivatives containing a pyrazole moiety. Annales Pharmaceutiques Françaises. 2016; 74 (6):431-438 - 81.
Krungkrai J, Krungkrai SR, Supuran CT. Malarial parasite carbonic anhydrase and its inhibitors. Current Topics in Medicinal Chemistry. 2007; 7 (9):909-917 - 82.
Maryam M, Tan SL, Crouse KA, Mohamed Tahir MI, Chee HY. Synthesis, characterization and evaluation of antidengue activity of enantiomeric Schiff bases derived from S-substituted dithiocarbazate. Turkish Journal of Chemistry. 2020; 44 (5):1395-1409 - 83.
Harpstrite SE, Collins SD, Oksman A, Goldberg DE, Sharma V. Synthesis, characterization, and antimalarial activity of novel Schiff Base-phenol and naphthalene-amine ligands. Medicinal Chemistry. 2008; 4 :392-395 - 84.
Fonkui TY, Ikhile MI, Njobeh PB, Ndinteh DT. Benzimidazole Schif base derivatives: Synthesis, characterization and antimicrobial activity. BMC Chemistry. 2019; 13 :127 - 85.
Devi J, Yadav J, Singh N. Synthesis, characterisation, in vitro antimicrobial, antioxidant and anti-inflammatory activities of diorganotin (IV) complexes derived from salicylaldehyde Schiff bases. Research on Chemical Intermediates. Jul 2019; 45 (7):3943-3968 - 86.
Pervaiz M, Ahmad I, Yousaf M, Kirn S, Munawar A, Saeed Z, et al. Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2019; (5) 206 :642-649 - 87.
Aggrawal S, Paliwal D, Kaushik D, Gupta GK, Kumar A. Pyrazole schiff base hybrids as anti-malarial agents: Synthesis, In vitro screening and computational study. Combinatorial Chemistry & High Throughput Screening. 2018; 21 :194-203 - 88.
Prakash CR, Raja S. Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. Journal of Saudi Chemical Society. Volume 17, Issue 3, July 2013, Pages 337-344. - 89.
Karrouchi K et al. Synthesis, antioxidant and analgesic activities of Schiff bases of 4-amino-1, 2, 4-triazole derivatives containing a pyrazole moiety. Annales Pharmaceutiques Françaises. 2016; 74 (6):421-438 - 90.
Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L. (2006). Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorganic & Medicinal Chemistry. 2006; 14 (11):3758-3765 - 91.
Murtaza S, Akhtar MS, Kanwal F, Abbas A, Ashiq S, Shamim S. Synthesis and biological evaluation of schiff bases of 4-aminophenazone as an anti-inflammatory, analgesic and antipyretic agent. Journal of Saudi Chemical Society. 2017; 21 :S359-S372 - 92.
Uddin N, Rashid F, Ali S, Tirmizi SA, Ahmad I, Zaib S, et al. Synthesis, characterization, and anticancer activity of Schiff bases. Journal of Biomolecular Structure and Dynamics. 2019; 2019 :1-14 - 93.
Abd-Elzaher MM, Labib AA, Mousa HA, Moustafa SA, Ali MM, El-Rashedy AA. (2016). Synthesis, anticancer activity and molecular docking study of Schiff base complexes containing thiazole moiety. Beni-Suef University Journal of Basic and Applied Sciences. 2016; 5 (1):85-96 - 94.
Tadele KT, Tsega TW. Schiff bases and their metal complexes as potential anticancer candidates: A review of recent works. Anti-Cancer Agents in Medicinal Chemistry. 2019; 19 (15):1786-1795 - 95.
Iacopetta D, Ceramella J, Catalano A, Saturnino C, Bonomo MG, Franchini C, et al. Schiff bases: Interesting scaffolds with promising antitumoral properties. Applied Sciences. 2021; 11 :1877 - 96.
Thomas AB, Nanda RK, Kothapalli LK, Hamane SC. Synthesis and biological evaluation of Schiff’s bases and 2-azetidinones of isonocotinyl hydrazone as potential antidepressant and nootropic agents. Arabian Journal of Chemistry. 2016; 9 (1):S79-S90 - 97.
Yuldasheva N, Acikyildiz N, Akyuz M, Dambagi LY, Aydin T. The synthesis of Schiff bases and new secondary amine derivatives of p-vanillin and evaluation of their neuroprotective, antidiabetic, antidepressant and antioxidant potentials. Journal of Molecular Structure. 2022; 1270 :133883 - 98.
Abdel-Wahab B, Mohamed S, Amr AG, et al. Synthesis and reactions of thiosemicarbazides, triazoles, and Schiff bases as antihypertensive α-blocking agents. Monatshefte fuer Chemie. 2008; 139 :1083-1090 - 99.
Gopi C, Sastry VG, Dhanaraju MD. Design, synthesis, spectroscopic characterization and anti-psychotic investigation of some novel Azo dye/Schiff base/chalcone derivatives. Egyptian Journal of Basic and Applied Sciences. 2017; 4 (4):270-287 - 100.
Udhayakumari D, Inbaraj V. A review on Schiff Base fluorescent Chemosensors for cell imaging applications. Journal of Fluorescence. 2020; 30 :1203-1223 - 101.
Liu H, Ding S, Lu Q, Jian Y, Wei G, Yuan A. A versatile Schiff Base Chemosensor for the determination of trace Co2+, Ni2+, Cu2+, and Zn2+ in the water and its bioimaging applications. ACS Omega. 2022; 7 :7585-7594 - 102.
Liu H, Liu T, Li J, Zhang Y, Li J, Song J, et al. A simple Schiff base as dual-responsive fluorescent sensor for bioimaging recognition Zn2+ and Al3+ in living cells. Journal of Materials Chemistry B. 2018; 6 :5435-5442 - 103.
Reyes JB, Flores BMM, Treviño AG, Suárez MAT, Hernández DP, Schott E, et al. Novel fluorescent Schiff bases as Al3+ sensors with high selectivity and sensitivity, and their bioimaging applications. Materials Chemistry and Physics. 2019; 233 :89-101 - 104.
Reyes JCB et al. Two-photon detection of organotin Schiff Base complexes in Cancer cells. Chemistry Selected. 2020; 5 (5):1623-1627 - 105.
Zhang J, Zhu X, Zhong A, Jia W, Wu F, Li D, et al. New platinum (II) one-armed Schiff base complexes for blue and orange PHOLEDs applications. Organic Electronics. 2017; 42 :153e162 - 106.
Gusev AN, Kiskin MA, Braga EV, Chapran M, Salyga GW, Baryshnikov GV, et al. Novel zinc complex with an ethylenediamine Schiff Base for high-luminance blue fluorescent OLED applications. Journal of Physical Chemistry C. 2019; 123 (18):11850-11859 - 107.
Nayak PHA, Naik HSB, Viswanath R, Kirthan BR. Green light emitting fluorescent [Zn(II)(Schiff base)] complexes as electroluminescent material in organic light emitting diodes. Journal of Physics and Chemistry of Solids. 2021; 159 :110288 - 108.
Berhanu AL, Gaurav MI, Malik AK, Aulakh JS, Kumar V, Kim KH. A review of the applications of Schiff bases as optical chemical sensors. Trends in Analytical Chemistry. 2019; 116 :74-91 - 109.
Xu J, Liu Y, Hsu SH. Hydrogels based on Schiff Base linkages for biomedical applications. Molecules. 2019; 24 :3005 - 110.
Liu J, Li J, Yu F, Zhao YZ, Mo XM, Pan JF. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. International Journal of Biological Macromolecules. 2020; 147 :653-666 - 111.
Oiyea EN, Ribeiroa MFM, Katayamaa JMT, Tadinia MC, Balbinoa MA, Eleoterio IC, et al. Electrochemical sensors containing Schiff bases and their transition metal complexes to detect analytes of forensic, pharmaceutical and environmental interest. A review. Critical Review in Analytical Chemistry. 2019; 49 (6):488-509 - 112.
Borisov SM, Pommer R, Svec J, Peters S, Novakova V, Klimant I. New red-emitting Schiff base chelates: Promising dyes for sensing and imaging of temperature and oxygen via phosphorescence decay time. Journal of Materials Chemistry C. 2018;6 :8999-9009 - 113.
Shakour MA, Said WA, Abdellah IM, · Su R, Shafei AE. Low-cost Schiff bases chromophores as efficient co-sensitizers for MH-13 in dye-sensitized solar cells. Journal of Materials Science: Materials in Electronics. 2019; 30 :5081-5091 - 114.
Jain P, Pandey G, Kumar D, Chandra S. Prospects of biologically active Schiff's base ligand and metal complexes in drug discovery. Advanced Science Engineering Medicine. 2019; 11 :144-154 - 115.
Chen G, Fang Y, Zhao X, Tat T, Chen J. Textiles for learning tactile interactions. Nature Electronics. 2021; 4 :175 - 116.
Basudeb D, Shibashis H. Schiff base compounds as fluorimetric pH sensor: A review. Analytical Methods. 2022; 22 :1-15