Abstract
A Schiff base is a compound with the general structure R1R2C=NR’. They can be considered a subclass of imines. The term Schiff base is normally applied when these compounds are used as ligands to form coordination complexes with metal ions. Schiff bases can be synthesised from a primary aliphatic or aromatic amine and a carbonyl compound by nucleophilic addition forming a hemiaminal, followed by a dehydration to generate an imine. In other words, a Schiff base is a nitrogen analogue of a ketone or aldehyde where the carbonyl group has been replaced by azomethine or imine group. The imine group present in these compounds has been shown to be critical to their biological activities. Schiff bases have been frequently used in various fields such as medicine, pharmaceutical purposes due to their wide range of industrial applications. The unconventional methods of preparation of Schiff bases, compared with traditional methods, are more convenient, and reactions can be carried out in higher yield, shorter reaction time and milder conditions, without generation of pollution and safer to analyse.
Keywords
- Schiff base
- synthesis
- imine
- carbonyl
- amine
- condensation
- solvent-free
- natural catalysts
1. Introduction
Schiff bases are versatile C=N containing compounds possessing broad range of biological activities, and incorporation of metals in form of complexes show some degree of antibacterial, antifungal, antitumor, antiviral and anti-inflammatory properties [1]. Schiff bases are typically formed by the condensation of a primary amine and an aldehyde or ketone. Structurally, a Schiff base is a nitrogen analogue of an aldehyde or ketone in which the carbonyl group has been replaced by an imine or azomethine group. Schiff bases are some of the most widely used organic compounds. They are used as pigments and dyes, catalysts, intermediates in organic synthesis, and as polymer stabilisers [2]. They are fundamental materials for the synthesis of various ligands which can be used as chiral auxiliaries in asymmetric synthesis. Metal complexes of Schiff bases have also been used in oxidation reactions [3].
Imine or azomethine groups are present in various natural, natural-derived and non-natural compounds. The imine group present in such compounds has been shown to be critical to their biological activities (Figure 1) [4, 5, 6]. Schiff bases are represented by the general formula R3R2C=NR1. The substituents R2 and R3 may be alkyl, aryl, heteroaryl or hydrogen while the substituent R1 at the
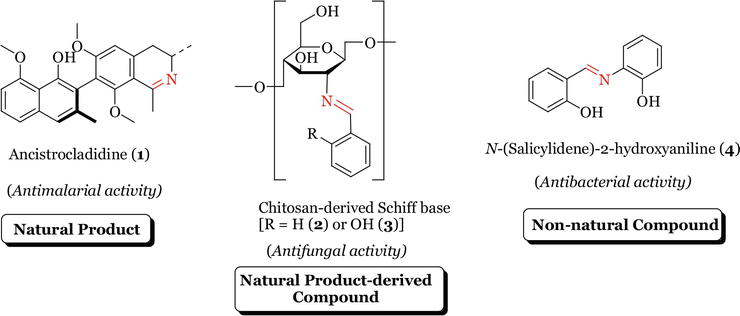
Figure 1.
Examples of bioactive Schiff bases.
The general approaches to the synthesis of Schiff bases are described in this report. Some unconventional methods of preparation and efficient practical techniques like microwave irradiation, solid-solid condensation, ultrasound irradiation, water suspension medium, infrared irradiation and the use of natural product as catalysts are discussed.
2. Preparation of Schiff bases
The most common method for the preparation of imines is the original reaction reported by Hugo Schiff in 1864 [7, 8, 9]. It is usually formed by the condensation of an aldehyde or ketone
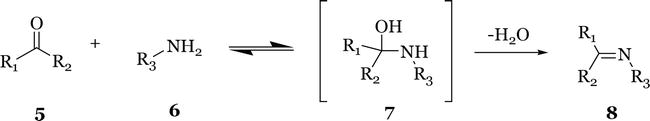
Figure 2.
Schiff reaction for the preparation of imines.
Aliphatic ketones react with amines to form imines more slowly than aldehydes; therefore, higher reaction temperatures and longer reaction time are required. Acid catalysts and water removal from the reaction mixture can significantly increase the reaction yields. Aromatic ketones are less reactive than aliphatic ones and require harsh conditions to be converted into imines.
Recently, a number of innovations and new techniques for the preparation of Schiff bases have been reported including solvent-free, clay, or microwave irradiation, solid-state synthesis, molecular sieves, liquid crystals, water suspension medium, infrared and ultrasound irradiation [11, 12, 13, 14, 15].
The mechanism of Schiff base formation is another variation of nucleophilic addition to the carbonyl group, where the nucleophile is the amine. The amine reacts with the carbonyl to give an unstable addition compound called carbinolamine. The carbinolamine loses water by either acid or base catalysed pathways. Since the carbinolamine is an alcohol, it undergoes acid catalysed dehydration (Figure 3) [16].
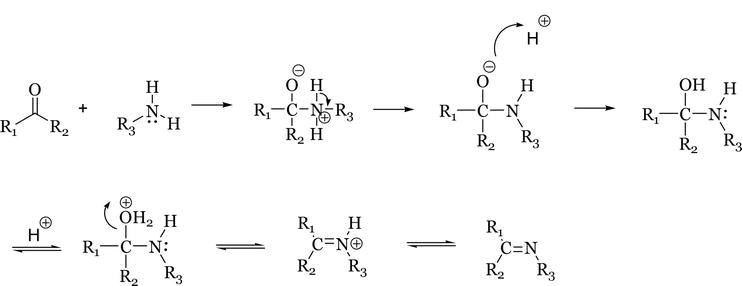
Figure 3.
Mechanism of formation of Schiff bases.
3. Synthesis of Schiff bases from various methods
The clay catalysed synthesis of imines and enamines under solvent-free conditions using microwave irradiation has been reported by Varma et al. [11]. A synthetic procedure catalysed by montmorillonite K 10 clay was employed for the preparation of imines and enamines. An equimolar mixture of the carbonyl compound and amine was placed in an open glass container and irradiated in a microwave oven at full power for 3 minutes. The product was extracted into DCM and removal of the solvent under reduced pressure gave the imines/enamines in 75–98% yield. This approach eliminates the need for the large excess of support usually employed in solid phase reactions and reduces considerably the longer times and large quantities of aromatic solvents required in the conventional solution phase chemistry which entails the azeotropic removal of water using Dean-Stark apparatus. This method was also employed by Vass et al. [17] for the synthesis of
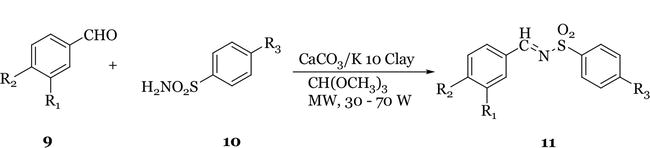
Figure 4.
Microwave-assisted synthesis of
The synthesis of imines has been carried out efficiently using silica gel supported sodium hydrogen sulfate (NaHSO4.SiO2), a non-toxic and inexpensive catalyst, as a reusable heterogeneous catalyst in solvent-free conditions under microwave irradiation [18]. Several substituted imines were prepared by this method (Figure 5). The NaHSO4.SiO2 catalyst can be reused by simple washing with diethyl ether after each use followed by activation in an oven at 120°C for 1 h prior to use, thus rendering the process more economical. This constitutes a green and efficient alternative to the MW assisted method described by Varma et al. [11] using K-10 clay as catalyst and the use of DCM or diethylether, which are the commonly used solvents for this reaction. The use of NaHSO4.SiO2 catalysed nucleophilic attack on the carbonyl group by the amine and served as a dehydrating agent to facilitate the removal of water in the final step. This eliminates the environmental disadvantages of using toxic drying agents such as TiCl4.
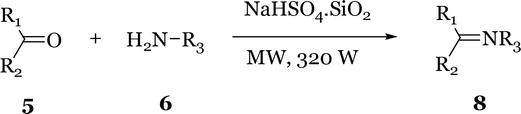
Figure 5.
Microwave-induced synthesis of imines using NaHSO4.SiO2 catalyst.
Schmeyers et al. [12] reported the solid-state synthesis of various kinds of benylideneaniline derivatives
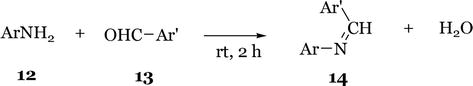
Figure 6.
Quantitative solid-solid synthesis of azomethines.
A mild and improved protocol for the preparation of imines by ultrasound irradiation has been developed by Guzen et al. [15]. A wide range of aromatic and heteroaromatic aldehydes were employed and all imines were obtained in excellent yields. The reaction was very efficient on a large scale, with the advantage of a very simple work-up and short reaction time (10 min). However, the process involved the use of a catalyst for activation and reaction solvent (Figure 7).
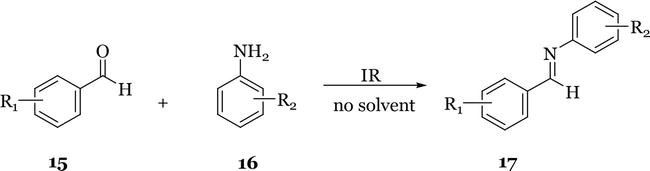
Figure 7.
Formation of
A new, simple, efficient, and environmentally benign method for the preparation of substituted
The synthesis of various kinds of imines by a simple and green procedure was reported by Tanaka and Shiraishi [13]. The process involved the condensation reactions of aldehydes and amines in a water suspension medium at room temperature. Water is a non-toxic, safe, and cheap medium. The use of water as solvent allowed the formation of imines without the need for catalysis, the use of a large excess of aromatic solvents, or the azeotropic removal of water. The reactions were completed in short reaction times, high-yielding and the products were isolated by filtration.
This procedure was also employed by Rao et al. [20] for the synthesis of Schiff bases via the condensation of 1,2-diaminobenzene with various substituted aromatic aldehydes in aqueous medium (Figure 8). The method was experimentally simple, clean, high-yielding, with reduced reaction times. The product was purified by simple filtration followed by washing with water and drying processes.
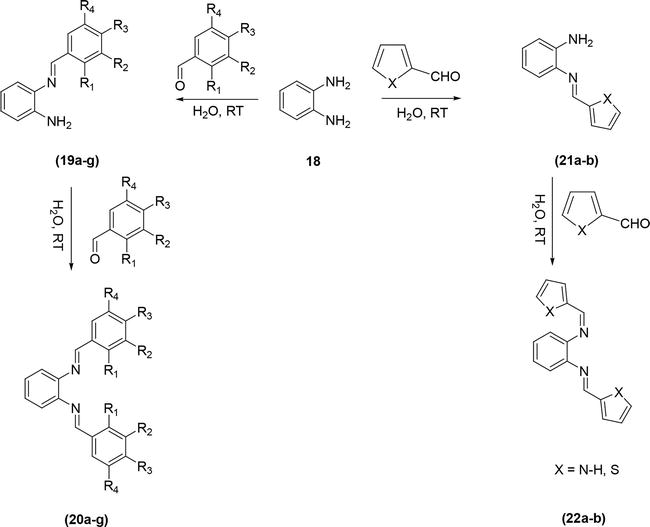
Figure 8.
Synthesis of Schiff bases in aqueous medium.
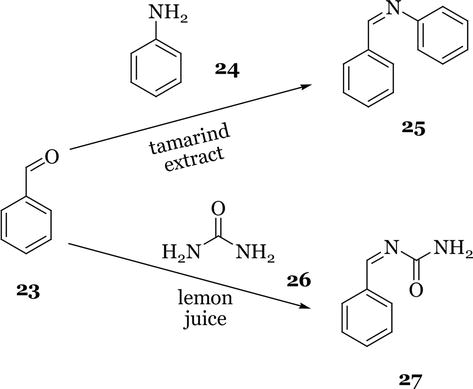
Figure 9.
Synthesis of Schiff bases catalysed by natural products.
4. Synthesis of Schiff bases using natural catalysts
A green method for the synthesis of Schiff bases using natural acids found in tamarind extract and lemon juice as a catalyst has been reported (Figure 9) [21]. The condensation of benzaldehyde
5. Conclusion
Some non-conventional methods of preparation of Schiff bases have been extensively discussed. These methods are environment friendly, free from organic solvents and without the drawbacks of long reaction time, special apparatus and cost of dehydrating agent.
References
- 1.
Gupta RR, Gupta V, Kumar M. Heterocyclic Chemistry. 1st ed. Berlin Heidelberg: Springer-Verlag; 1998 - 2.
da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CVB, et al. Schiff bases: A short review of their antimicrobial activities. Journal of Advanced Research. 2011; 2 :1-8 - 3.
Jarrahpour AA, Rezaei S. Synthesis of N,N'-bis (a-methylsalicylidene) 4,4′-diaminophenylmethane as a novel complexing agent. Molbank. 2006; 2006 (1):M456 - 4.
Bringmann G, Dreyer M, Faber JH, Dalsgaard PW, Jaroszewski JW, et al. Ancistrotanzanine C and related 5,1′- and 7,3'-coupled Naphthylisoquinoline alkaloids from Ancistrocladus tanzaniensis. Journal of Natural Products. 2004; 67 (5):743-748 - 5.
de Souza AO, Galetti FCS, Silva CL, Bicalho B, Parma MM, Fonseca SF, et al. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quimica Nova. 2007; 30 (7):1563-1566 - 6.
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, et al. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quartenized chitosan. Carbohydrate Research. 2007; 342 (10):1329-1332 - 7.
Schiff H. Mittheilungen aus dem universitatslaboratorium in Pisa: Eine neue reihe organischer basen. Justus Liebigs Annalen der Chemie. 1864; 131 :118-119 - 8.
Schiff H. Eine neue reihe organischer diamine. Justus Liebigs Annalen der Chemie. 1866; 140 :92-137 - 9.
Schiff U. Giornale di scienze naturali ed economiche. Palermo. 1867; II :1-59 - 10.
Taguchi K, Westheimer FH. Catalysis by molecular sieves in the preparation of ketimines and enamines. The Journal of Organic Chemistry. 1971; 36 :5556-5557 - 11.
Varma RS, Dahiya R, Kumar S. Clay catalyzed synthesis of imines and enamines under solvent-free conditions using microwave irradiation. Tetrahedron Letters. 1997; 38 :2039-2042 - 12.
Schmeyers J, Toda F, Boy J, Kaupp G. Quantitative solid-solid synthesis of azomethines. Journal of the Chemical Society, Perkin Transactions. 1998; 2 :989-993 - 13.
Tanaka K, Shiraishi R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chemistry. 2000; 2 :272-273 - 14.
Andrade CKZ, Takada SCS, Alves LM, Rodrigues JP, Suarez PAZ, Brandao RF, et al. Molecular sieves in ionic liquids as an efficient and recyclable medium for the synthesis of imines. Synlett. 2004; 12 :2135-2138 - 15.
Guzen KP, Guarezemini AS, Orfao ATG, Cella R, Pereira CMP, Stefani HA. Eco-friendly synthesis of imines by ultrasound irradiation. Tetrahedron Letters. 2007; 48 :1845-1848 - 16.
Xavier A, Srividhya N. Synthesis and study of Schiff base ligands. IOSR-JAC. 2014; 7 :6-15 - 17.
Vass A, Dudas J, Varma RS. Solvent-free synthesis of N-sulfonylimines using microwave irradiation. Tetrahedron Letters. 1999; 40 :4951-4954 - 18.
Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R. Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. Journal of Chemistry Research. 2005; 5 :299-303 - 19.
Vazquez MA, Landa M, Reyes L, Miranda R, Tamariz J, Delgado F. Infrared irradiation: Effective promoter in the formation of N-Benzylideneanilines in the absence of solvent. Synthetic Communications. 2004; 34 :2705-2718 - 20.
Koteswara Rao V, Subba Reddy S, Satheesh Krishna B, Reddi Mohan Naidu K, Naga Raju C, Ghosh SK. Synthesis of Schiff’s bases in aqueous medium: A green alternative approach with effective mass yield and high reaction rates. Green Chemistry Letters and Reviews. 2010; 3 :217-223 - 21.
Wahab A, Haider SS, Mahmood I, Mahmood T, Sherwani SK, Kanwal S. Synthesis of Schiff bases from natural products and their remarkable antimicrobial and antioxidant activity. FUUAST Journal of Biology. 2014; 4 :27-32