Different analytical parameters for the analysis of heavy metal ions using Schiff bases.
Abstract
The Schiff base was first synthesized by Hugo Schiff through the condensation reaction of primary amines with carbonyl compounds (aldehyde or ketone) in 1864. Schiff bases exhibit many structural and electrical characteristics that enable their use in a variety of fields, including medical and chemosensing. Schiff bases generate stable complexes when they bind with different metal ions. Schiff bases are employed as fluorescent turn-on/turn-off chemosensors for the detection of various metal cations, such as Hg2+, Cd2+, Cr3+, Pd2+, and As3+ in various materials due to their outstanding coordination ability. This chapter examines a variety of Schiff bases that are employed in chemosensing procedures for various metal ions (such as divalent and trivalent cations) in various biological, agricultural, and environmental settings.
Keywords
- thiosemicarbazones
- chemosensor
- fluorescence
- copper
- fluoride ion
1. Introduction
Various fields use metallic cations, some of which control countless biological processes necessary for life. However, since they are not biodegradable and can accumulate in the food chain, the excess of these ions can lead to serious environmental issues. Even at low concentrations, they constitute a substantial threat to both the environment and human health. Numerous health issues, such as allergies, lung damage, anemia, kidney failure, neurotoxicity, genotoxicity, oxidative toxicity, steroidogenic toxicity, sperm toxicity, apoptotic toxicity, and axillary toxicity, can be brought on by these ions [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. So it is crucial to architect an effective method for identifying these ions in various samples. In order to detect metallic cations, different techniques have been developed, including liquid chromatography [11], electrochemistry [12], voltammetry [13], reversed-phase high-performance liquid chromatography [14], and inductively coupled plasma-mass spectrometry [15]. Even though these methods are quite effective, they nevertheless have certain drawbacks, such as being expensive, difficult to use, requiring a lot of sample preparation, and providing ambient conditions for their use. As an alternative to the aforementioned drawbacks of conventional techniques, fluorescent-based Schiff bases have been developed for the detection of metallic cations. They serve as stabilizers for polymers, catalysts, pigments, and dyes, intermediates in organic synthesis, and pigments and dyes [16]. Additionally, a variety of biological activities involving antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral, and antipyretic effects have been linked to Schiff bases [17, 18]. There are imine or azomethine groups in a variety of natural, natural-derived, and synthetic substances. It has been demonstrated that these compounds’ imine group is essential for their biological actions. Schiff bases with great sensitivity and selectivity to a variety of species (cations and anions) were demonstrated by the spectrofluorometric method. In particular, chemosensors based on Schiff bases have shown exceptional performance for the identification of different metallic cations due to their simple and affordable production as well as their capacity to coordinate with practically all metal ions and stable them in a variety of oxidation states. Additionally, these Schiff bases have demonstrated a wide range of biological uses, such as anti-ulcerogenic, analgesic, antifungal, anti-inflammatory, anti-viral, antioxidant, and anticancer effects. In various processes, many of these compounds exhibit outstanding catalytic efficiency [19, 20]. Various Schiff bases have been examined in this review article as fluorescent turn-on/turn-off chemosensors for the detection of different metal ions in various matrices. Additionally, many Schiff base chemosensors’ synthetic processes and sensing mechanisms have been studied.
2. Synthesis of Schiff bases
Any primary amine can produce Schiff bases under specified conditions when it interacts with an aldehyde or ketone. In terms of structure, a Schiff base is an aldehyde or ketone that has had the carbonyl group (C=O) substituted by an imine or azomethine group, creating a nitrogen analogue. The synthesis of Schiff bases has used a variety of synthetic methods. Only a few straightforward synthetic methods are described here for the benefit of beginners and others who are unfamiliar with organic synthetic chemistry. Hugo Schiff (1864) was the first to produce Schiff bases using an azeotropic distillation reaction between primary amines and an aldehyde or ketone (Compound a, Figure 1) [21]. Catalysts of many types, such as acetic acid, p-toluene sulfonic acids, montmorillonite, and acid resin. There have been reports of the synthesis of Schiff bases from primary amines and carbonyl compounds (aldehyde or ketone) using resin, HCl, H2SO4, TiCl4, Mg(ClO4)2, SnCl4, BF3Et2O, ZnCl2, MgSO4, etc. Additionally, under green chemistry circumstances, microwave irradiation of Schiff bases has been modified for solvent-free synthesis [22, 23]. In this approach, aldehyde or ketone-containing carbonyl compounds react with primary amines to produce Schiff bases with a high yield (Compound b, Figure 1). The process can proceed at ambient by the use of an appropriate catalyst. Without the use of any solvent, the reactants (primary amines and aldehyde/ketone) are ground in a mortar and pestle (Compound c, Figure 1) [24]. Additionally, primary amines and alcohol can be combined to create a Schiff base (Compound d, Figure 1). This approach works with a variety of alcohols and amines and does not need any additional equipment or supplements [25].
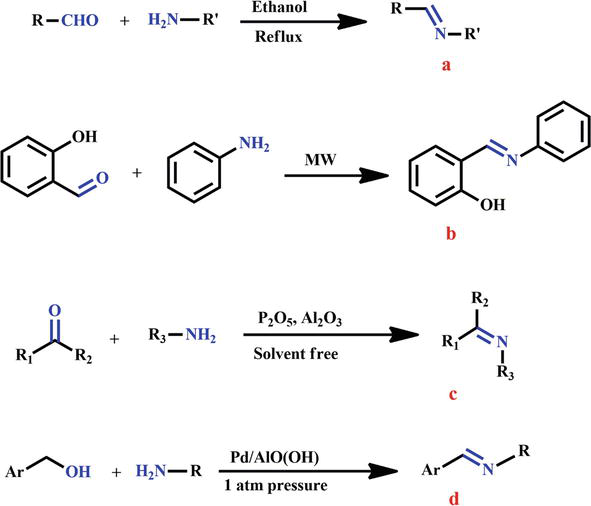
Figure 1.
The synthesis of Schiff base derivatives via several synthetic pathways.
3. Chemosensors using Schiff bases to find hazardous metal ions
For fluorescence turn-on/turn-off detection of different metal cations, a number of chemosensors based on Schiff bases have been created and employed (Table 1). Before employing these compounds as chemosensors, there are a few ideas to keep in mind. In order to create a heterogeneous system in an aqueous medium, bulky chains are preferred. This makes it possible to set up a system for quick and affordable separation. Additionally, spectroscopic techniques are used in the field of chemosensing; hence, the Schiff bases should preferably comprise a fluorophore for fluorescent spectroscopic studies [26]. Similar to this, a spacer (which can change the geometry of the system and control the electrical interaction between the receptor and chromophore unit) and a receptor (which is responsible for the selective analyte binding) are needed [27].
| Sensor | Sensor type | Sensing mechanism | Analyte | M: L | LOD | Wavelength λex/λem (nm) | pH range | Matrices | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Turn on | Chelation & CHEF | Hg2+ | 2:1 | 2.82 μM | 347/445 | 6–8 | Living cells | 38 |
| 2 | Turn on | — | Hg2+ | 1:2 | 75 μM | 369/464 | 8 | Ethanol/H2O | 39 |
| 3 | Turn off | PET | Hg2+ | 1:2 | 8.3 nM | 420/478 | 3 | HEPES buffer | 40 |
| 4 | Turn on | — | Hg2+ | 1:1 | 55.95 nM | 305/440 | — | DMSO | 41 |
| 5 | Turn off | ICT | Hg2+ | 1:1 | 25.2 nM | 370/436 | — | can | 42 |
| 6 | Turn on | ESIPT | Hg2+ | 1:2 | 22.6 nM | 349/473 | 8 | H2O/AcN | 43 |
| 7 | Turn on | CHEF | Cd2+ | 1:2 | 14.8 nM | 456/510 | — | H2O/AcN | 44 |
| 8 | Turn on | PET/CHEF | Cd2+ | 1:1 | 0.12 μM | 380/506 | 5-9 | can | 45 |
| 9 | Turn on | FRET | Cd2+ | 1:1 | 10.25 nM | 308/590 | 3-9 | can | 46 |
| 10 | Turn on | — | Cd2+ | 1:1 | 60 μM | 385/474 | 8 | Ethanol/H2O | 47 |
| 11 | Turn on | CIFA | Cd2+ | 1:1 | 0.218 μM | 530/591 | — | H2O | 48 |
| 12 | Turn on | PET | Cr3+ | 1:1 | 1.5 μM | 320/562 | 8 | H2O/THF | 49 |
| 13 | Turn on | Dissociation | Cr3+ | 1:2 | 0.94 nM | 327/418 | 5-10 | Ethanol | 50 |
| 14 | Turn off | IFE | Cr6+ | 1:1 | 0.175 μM | 350/449 | — | Ascorbicacid/ H2O | 51 |
| 15 | Turn on | PET | Cr3+ | 1:2 | 0.5 μM | 279/568 | 6-10 | MeOH/H2O | 52 |
| 16 | Turn on | PET | Cr3+ | 1:1 | 13 μM | 360/663 | 9 | H2O/AcN | 53 |
| 17 | Turn on | Chelation | Pd2+ | 1:1 | 0.05 μM | 485/563 | 7.4 | living cells HCT116 | 54 |
| 18 | Turn on | Chelation | Pd2+ | 1:1 | 0.018 μM | 450/598 | 7–12 | living cells MCF7 | 55 |
| 19 | Turn on | ESIPT | As3+ | 1:1 | 69.7 nM | 410/525 | 1-13 | Naphtha/Ethanol | 59 |
| 20 | Turn on | — | As3+ | 1:1 | 7.2 ppb | 350/− | 6-8 | H2O | 60 |
| 21 | Turn on | — | As3+ | 1:1 | 0.45 μM | 385/496 | — | THF | 61 |
| 22 | Turn on | Chemodosimetry | Au3+ | 1:1 | 1.51 μM | 560/588 | — | H2O/AcN | 62 |
| 23 | Turn on | AIE | Y3+ | 1:1 | 22.3 μM | 360/575 | 4-8 | THF/H2O | 63 |
| 24 | Turn on | ICT | Y3+ | 1:1 | 0.013 ppb | 435/495 | — | THF | 64 |
| 25 | Turn on | PET | Ga3+ | 1:1 | 0.01 μM | 300/434 | — | DMF/AcN | 65 |
| 26 | Turn on | PET | Ga3+ | 1:2 | 3.90 nM | 352/421 | — | H2O/AcN | 66 |
| 27 | Turn on | PET | Ga3+ | 1:2 | 3.97 nM | 352/421 | — | H2O/AcN | 67 |
Table 1.
4. The sensing mechanism of the Schiff base chemosensor, including its selectivity and sensitivity
The electron configuration of both the ligand and the metal ion, as well as the structural stiffness and the capacity of the Schiff base chemosensor to attach to the metal ion, is a parameter that controls the selectivity and high sensitivity of Schiff base chemosensors [28, 29]. Therefore, various mechanisms, such as chelation-enhanced fluorescence (CHEF), photo-induced electron transfer (PET), intra/inter-molecular charge transfer (ICT), hydrolysis, chelation enhancement quenching effect (CHEQ), and ring-opening mechanisms, have been proposed for the interaction between metal ions and various chemosensors. In this chapter, we also briefly address the mechanisms underlying the chemosensory applications of Schiff base derivatives. (i) Electron transfer (ET): Out of all of these traditional processes, ET-primarily via photo-induced electron transfer (PET)—has been the one used the most frequently in fluorescent chemosensors. A fluorophore’s fluorescence is typically quenched by the PET process; however, it may be restored if guest molecules can prevent the PET process. (ii) Charge transfer (CT): ICT (intramolecular charge transfer), MLCT (metal-ligand charge transfer), and twisted ICT are examples of CT processes (TICT). A ratiometric signal is produced by an ICT-based chemosensor when an ICT process is enhanced or suppressed. This ratiometric signal can remove the majority of ambiguities by self-calibrating two emission bands and enable quantitative determination in more advanced applications, such as imaging in living cells and tissues. On the other hand, transition metal complexes like those of ruthenium, rhenium, and iridium, etc., frequently exhibit MLCT, in which CT occurs from a ligand to a transition metal cation. Through the influence of MLCT energy level by analytes, it can also be employed for chemosensor design. Additionally, TICT is a potent intramolecular CT that takes place in the excited state and requires solvent relaxation surrounding the molecule to produce an ongoing rotation of the electron donor and acceptor until it is twisted roughly 90 degrees [30, 31]. Since intramolecular rotation and charge separation in the TICT state depend on polar solvent relaxation, the fluorescence behavior is extremely sensitive to micropolarity and/or steric barrier for molecular rotation [29, 32, 33]. (iii) Energy transfer (ET): Depending on the interaction distance between the energy donor and energy acceptor, ET can be divided into two categories: electronic energy transfer (EET) and fluorescence resonance energy transfer (FRET). While FRET requires a specific amount of spectral overlap between the emission spectrum of the donor and the acceptor, EET, also known as Dexter electron transfer, requires a distance between the donor and acceptor of less than 10 to be effective. For effective FRET to happen, the distance between the donor and acceptor needs to be between 10 and 100. As a result, the chemosensors based on the energy transfer mechanism are highly dependent on distance. (iv) Excimer/exciplex: An excimer is a complex that is created when a fluorophore interacts with another fluorophore of the same structure in the ground state while it is stimulated. The resulting complex is known as an exciplex if the fluorophore in the excited state differs from the fluorophore in the ground state. A dual emission from the monomer and excimer/exciplex is frequently recorded at the same time because the emission spectra of an excimer/exciplex are red-shifted in comparison to that of the monomer. The excimer/exciplex band can therefore be monitored to detect excimer/exciplex production or deformation in response to interaction with a guest species. However, there are currently just a few examples of chemosensors that work by forming exciplexes [32, 34, 35].
5. Detection of some toxic heavy metal ions
5.1 Detection of mercury
Mercury is a toxic heavy metal even at low concentrations and widely dispersed in nature. It can accumulate in the body and is responsible for different diseases including acute kidney failure, prenatal brain damage, and heart diseases. Therefore, the detection of Hg2+ ions in different samples is of great consideration. A novel Schiff base chemosensor 1 containing pyrene-based free thiol derivative was used for the determination of Hg2+ ions in living cells [36]. Therefore, it is quite important to consider finding Hg2+ ions in various samples. For the detection of Hg2+ ions in live cells, a new Schiff base chemosensor 1 with pyrene-based free thiol derivative was employed. Similar to this, a double-naphthalene Schiff base fluorescent chemosensor 2 was used to detect Hg2+ ions at a low detection limit of 0.05595 μM in a DMSO medium (Figure 2). In the presence of DMSO, the chemosensor’s imine group underwent oxidation to become an amide. The 1:1 metal-ligand complex generated by the N- and O- atoms of amide and Hg2+ ions was confirmed by mass spectrometry, 1H NMR, and FT-IR spectroscopy [37]. A novel coumarin-based fluorescent chemosensor 3 was employed in another study to find Hg2+ ions. In the presence of secondary metal ions, the chemosensor shows excellent selectivity toward Hg2+. As the amount of Hg2+ gradually increases, the probe’s yellow-colored solution becomes colorless. Fluorescence investigations further supported chemosensor 3’s selectivity for mercury ions. The bright blue fluorescence at 460 nm gradually decreases in conjunction with a minor red-shift to the cyan channel (470 nm) after the addition of 0.4 M Hg2+ to the probe solution. The lone pair electrons are distributed to Hg2+ by the Schiff base imine nitrogen (CH=N) and phenolic-oxygen atoms, which reduces the emission of acceptor coumarin and prolongs that of the donor 4-(Diethylamino)salicylaldehyde. Therefore, 4-(Diethylamino)salicylaldehyde’s distinctive emission at 470 nm is due to the inhibition of the ESIPT and PET-ON processes. The mole fraction at maximum absorbance is 0.3, showing that Cou-S and both Hg2+ form a dimer complex with a binding stoichiometry of 2:1 (probe: metal ion). An 8.3 nM threshold for detection was determined. Density functional theory (DFT) was also used to analyze how the chemosensor and Hg2+ ions interact [38]. Another work used a novel N-salicylidene)benzylamine Schiff base 4 to detect Hg2+ ions via fluorescence ON characteristics. Fluorescence and UV-Vis spectroscopy were used to examine the chemosensor’s sensing capabilities. By using fluorescence emission and UV-Vis spectroscopy, it was determined how the chemosensor 4 responded to different cations. Significant fluorescence enhancement and spectral/color alterations supported the sensor’s selectivity for Hg2+ [39]. The detection of Hg2+ ions is influenced by the pH of the detection medium. For example, a turn-on fluorescent chemosensor 5 was developed to detect Hg2+ in an aqueous CH3CN medium with a pH range of 5–10 [40]. In the presence of various cations including Hg2+, Pb2+, Ca2+, Ba2+, Cu2+, Cr3+, Mg2+, Zn2+ ions in aqueous media, it was discovered that the Hg2+ ion notably exhibited specificity for the Schiff base chemosensor 6. Additionally, counter anions such as Br−, Cl−, F−, I−, S2−, CN−, NO3−, NO2−, OH−, CO3−, SCN−, HSO3−, H2PO4− CH3COO−, HPO42−, P2O74−, and PO43− failed to have a discernible impact on the chemosensor’s selectivity [41]. The structures of the chemosensors 1–6 and their interaction with Hg2+ ion are shown in Figure 2.
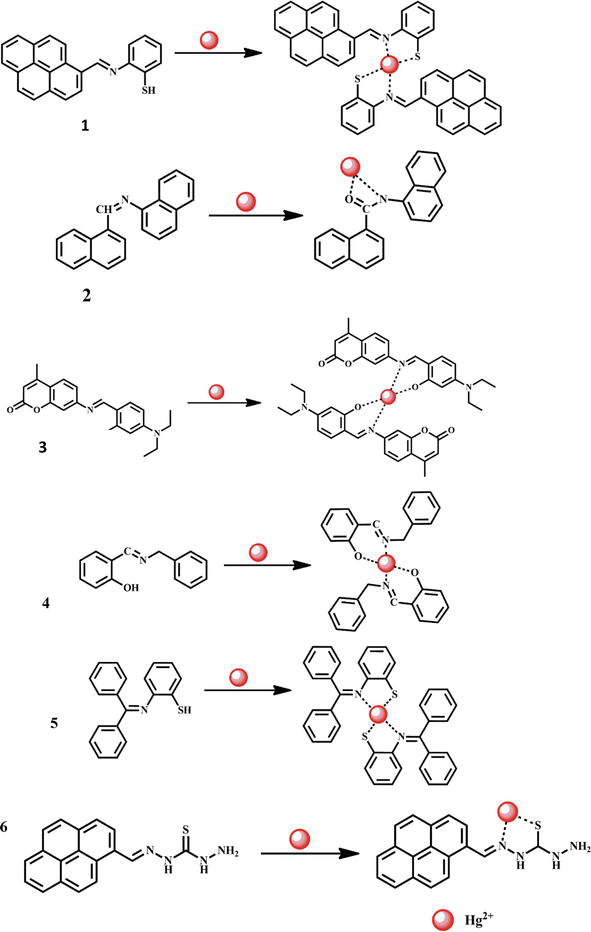
Figure 2.
Formation of Hg2+ complexes with Schiff base chemosensors 1–6.
5.2 Detection of cadmium ion
Due to the extensive range of uses of cadmium in numerous industries, including mining, smelting, and the combustion of fossil fuels, etc., it is one of the most pervasive health-hazardous contaminants. In light of this, it is crucial to create a reliable and efficient method for detecting Cd2+ ions in a variety of materials. The quinoline moiety-containing Schiff base chemosensor 7 was created as a fluorescence sensor for Cd2+ ions. It’s interesting to note that the chemosensor performed superbly when detecting Cd2+ ions in an aqueous media by turn-on fluorescence. Very little fluorescence emission was seen from chemosensor 7 when it was unmodified, but a significant turn-on response was seen when Cd2+ was added. The sensor showed excellent selectivity with no interference from any other metal ions. The limit of detection was determined to be 0.0024 μM, and the binding stoichiometry between the Cd2+ and chemosensor 7 was proven to be 1:1 [42]. To identify Cd2+ ions, a pyridine-based Schiff base chemosensor 8 was created. The fluorescence enhancement happened when the Cd2+ ion was applied to the complexion despite the chemosensor not being fluorescent. As a result, the CHEF was stimulated while the PET was blocked, increasing the fluorescence intensity of the chemosensor. Additionally, chemosensor 8 was successfully used in water sample analysis [43]. With a detection limit of 1.025 × 10−8 M, a Rhodamine-based Schiff-base fluorescence chemosensor 9 was used to find Cd2+ ions. In the presence of cations, the chemosensor 9 shows significant sensitivity and selectivity for Cd2+ ions [44]. An ON-type Schiff base chemosensor 10 was communicated in 2020 and its fluorescence properties against cadmium ions were discussed. According to Job plot, the chemosensor creates a complex with Cd2+ of 1:1 ratio. For an analytical approach based on chemosensor, pH, solvent type, and ligand concentration were tuned for the detection of Cd2+ in aqueous samples. The limit of detection was determined to be 6.0 × 10−7 M. The probe showed a wide range of linearity with Cd2+ [45]. A recent study described fluorescent sensor 11, which is based on rhodamine, and its capacity to find Cd2+ ions. The coordination-induced fluorescence activation (CIFA) mechanism is how the sensor reacts to Cd2+. In the presence of the tested metal ions, chemosensor 11 responds to Cd2+ with a highly quick and reversible fluorescence. The complex stoichiometry between the sensor and Cd2+ was discovered to be 1:1, and the binding constant in acetonitrile (ACN)/HEPES buffer (10 mM, pH, 7.05, v/v 1:1) was calculated to be 2.70 107 M−1. The chemosensor 11’s fluorescence detection limit for Cd2+ was discovered to be 0.218 μM, demonstrating a notable sensitivity to Cd2+ [40]. The structures of the chemosensors 7–11 and their interaction with Cd2+ ion are shown in Figure 3.
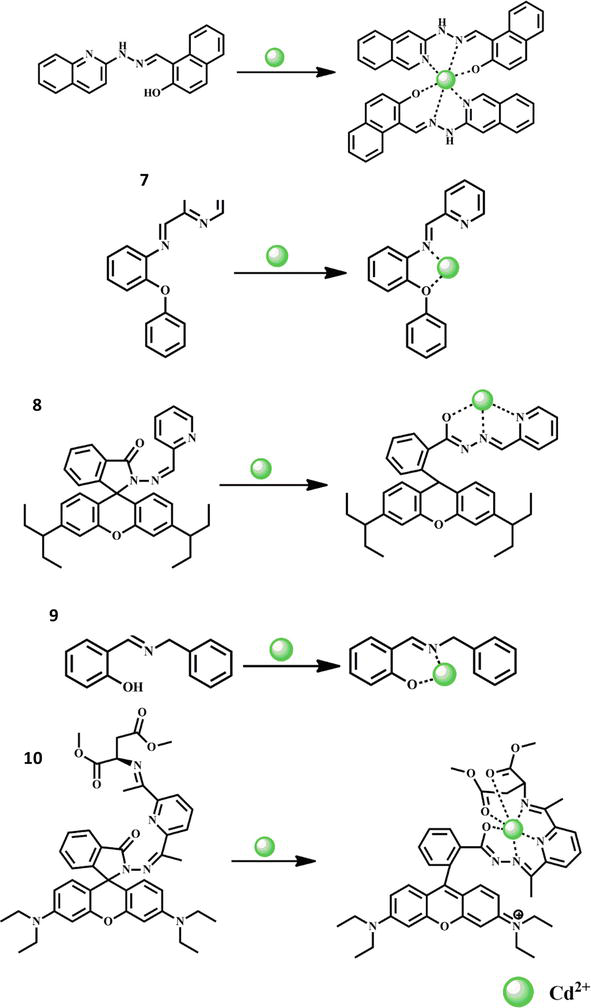
Figure 3.
Formation of Cd2+ complexes with Schiff base chemosensors 7–11.
5.3 Detection of chromium ion
Chromium is a significant transition metal that is used in a variety of industries, including chemical engineering, textile manufacturing, steel production, oil refining, and electroplating. But it raises the risk of lung, sinus, and nasal cancer and results in pulmonary sensitization. A novel Schiff base chemosensor 12 was constructed and subjected to several characterization methods in order to detect Cr3+. Using a spectrophotometric method, the chemosensor was tested against a variety of toxic metal ions, including Mn2+, Ag+, Cd2+, Co2+, Cu2+, Ni2+, Zn2+, Fe3+, Al3+, and Cr3+. Only Cr3+ ions were found to produce a recognizable hyperchromic shift in the chemosensor’s absorbance due to the formation of the stable complex through S and N atom [46].
Additionally, electrochemical methods were employed to investigate the electrochemical properties and reversibility of the chemosensor. To detect Cr3+ ions in the aqueous media, the Schiff base chemosensor 13 was also used. Visual monitoring was done of the chemosensor 13’s colorimetric sensitivity to several cations. When chromium ions were added, the chemosensor 13’s yellow color turned to colorless. Fluorescence tests were used to investigate the chemosensor 13’s sensing capabilities in more detail. The chemosensor 13 displayed less fluorescence intensity at 418 nm, but a significant increase in fluorescence intensity was seen in the presence of Cr3+ due to the imine group of sensor might be converted back to the carbonyl group in the presence of Cr3+ [47]. Recently, a novel Schiff base fluorescent chemosensor 14 for the selective detection of chromium ions was published. The development of a 1:1 stoichiometric sensor-Cr3+ complex, which is supported by Job’s plot, has the effect of inhibiting the PET process. The limit of detection was shown to be 1.3 × 10−7 M via fluorescence titration, and the association constant Ka was reported as 2.28 × 105 M−1. Additionally, DFT and TDDFT simulation results were provided to understand the proposed complex’s optimal structure as well as its electronic spectra [48]. An innovative thiazole-based fluorescent Schiff base chemosensor 15 for the chemodosimetric method to Cr(III) ion detection was discussed. The structure of the chemosensor 15 was determined using a variety of analytical techniques, including UV-vis, 13C-NMR, 1H-NMR, and FT-IR analysis. It’s interesting to note that chemosensor 15 responds to different metal cations, such as Ni2+, Na+, Cd2+, Ag+, Mn2+, K+, Zn2+, Cu2+, Hg2+, Co2+, Pb2+, Mg2+, Sn2+, Al3+, and Cr3+, by turning on fluorescently to specific Cr(III) ions [49]. Whereas an inner filter effect-based Schiff base chemosensor 16 was adequately quenched by Cr(VI) through primary and secondary inner filter effects. The addition of L-ascorbic acid in the concentration range of 10 μM to 390 μM with an LOD of 2.46 μM efficiently turned on the chemosensor-Cr(VI) solution’s switched-off fluorescence. The reduction of Cr(VI) to Cr(III) by L-ascorbic acid led to the elimination of both primary and secondary inner filter effects and the recovery of the chemosensor’s fluorescence, which is the mechanism proposed for the fluorescence turn-on of the Cr(VI)-chemosensor’s quenched fluorescence [50]. The structures of the chemosensors 12-15 and their interaction with Cr3+ ion are shown in Figure 4.
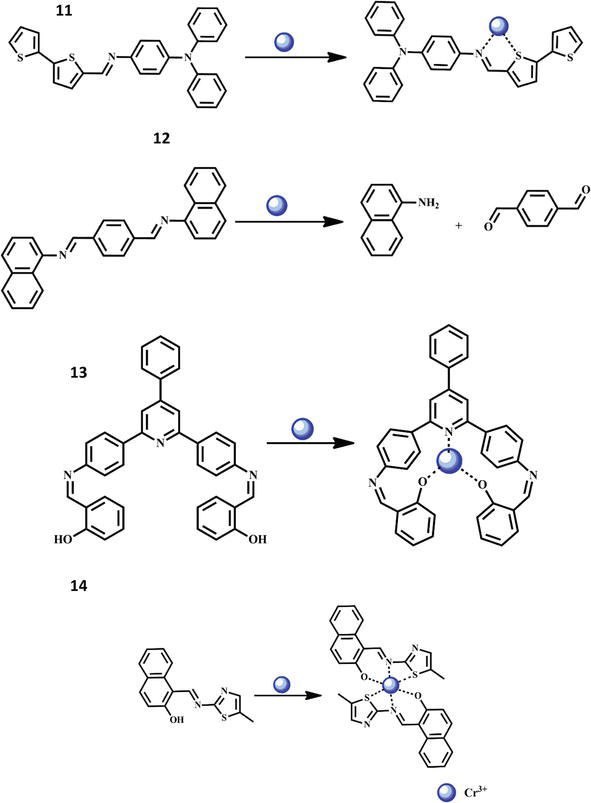
Figure 4.
Formation of Cr3+ complexes with Schiff base chemosensors 12–16.
5.4 Detection of palladium ion
A valuable metal, palladium is used in several industrial and electronic devices. However, palladium is extremely carcinogenic and poisonous. The detection of Pd2+ ions in various environmental, biological, and agricultural materials is therefore crucial. For the purpose of detecting Pd2+ ions, the Schiff base fluorescent and colorimetric chemosensor 17 was employed. High selectivity toward Pd2+ ions was demonstrated by this chemosensor in the presence of monovalent, divalent, trivalent, and tetravalent cations such as Ba2+, Cu2+, Zn2+, Fe2+, Tb3+, Hg2+, Eu3+, Mg2+, Gd3+, Mn2+, Sm3+, Cr3+, Ag+, Ca2+, Sn4+, and Ni2+. Mass, 1H NMR, 13C NMR, and FT-IR analyses all validated the spirolactam ring opening as the chemosensor’s mechanism. Chemosensor 17’s Pd2+ detection threshold was established at 0.05 μM [51]. In the presence of the ions Ba2+, Cu2+, Zn2+, Al3+, Fe2+, Hg2+, Mg2+, Co3+, Mn2+, Ag+, Ca2+, Ni2+, Cd2+, Pb2+, Fe3+, Pt2+, Na+, and K+, Coumarinyl-rhodamine Schiff base chemosensor 18 functions as an incredibly selective chemosensor for Pd2+. The spirolactam ring being opened has been suggested as the mechanism for this chemosensor. The Pd2+ ions improve the fluorescence intensity and change the color of the chemosensor from straw color to pink, whereas the free chemosensor was weakly emissive. In the presence of chemosensor 18 and distinct metal cations, this chemosensor was highly selective for the Pd2+ ion [52]. The structures of the chemosensors 17–18 and their interaction with Cr3+ ion are shown in Figure 5.
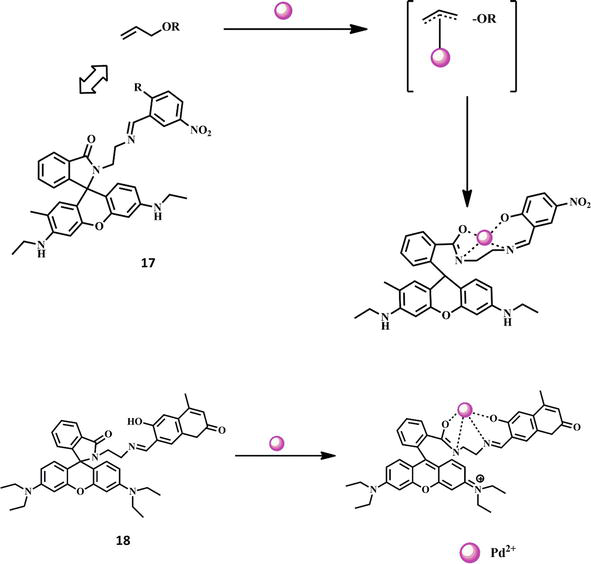
Figure 5.
Formation of Pd2+ complexes with Schiff base chemosensors 17–18.
5.5 Detection of Arsenic ion
Despite being extensively distributed in nature, arsenic is a deadly heavy metal even at low amounts. It builds up in the body and causes a variety of illnesses, such as lung and skin cancers, interference with cellular respiration, and affects the functions of the liver, kidney, bladder, and prostate [53, 54, 55]. Therefore, the detection of As3+ ions in different samples is of great consideration. A recent study described the detection of arsenic species found in a real naphtha sample using a fluorescence chemosensor 19 based on excited-state intramolecular proton transfer (ESIPT). The ESIPT process of M-HBT was demonstrated by the pH-related emission migration from 610 nm (pH 14,1) to 510 nm (pH 14,13). The sensing ability of M-HBT was examined in ethanol, and the experimental findings showed that As3+ responded linearly well in the range of 0–32 μM [56]. Similar to this, for the quick detection of As3+/As5+ in mixed aqueous solutions, a novel colorimetric sensor based on a benzothiazole Schiff base chemosensor 20 has been reported. It offers advantages of simplicity, specificity, high sensitivity, selectivity, and economy with a detection limit of 7.0 ppb within 10 s via the naked eye. Moreover, the probe is appropriate for on-site, quick, and convenient detection of As3+/As5+ ions at extremely low concentrations in actual water samples and exhibits remarkable selectivity in the presence of other common ions [57]. A novel coumarin-based fluorescent chemosensor 21 was employed in another study to find As3+ ions. In the presence of secondary metal ions (Na+, Mg2+, Cu2+, Ni2+, Co2+, Zn2+, Pb2+, Fe2+, Fe3+, Hg2+, Ca2+, Cd2+, and Mn2+), the chemosensor shows excellent selectivity toward As3+. The development and evaluation of ArsenoFluor1, the first example of a chemosensor was done for the detection of As3+ in organic solvents at 298 K. At λem = 496 nm in THF, AF1 shows a 25-fold increase in fluorescence that is selective for As3+ over other physiologically relevant ions (such as Na+, Mg2+, Fe2+, and Zn2+) and has a sub-ppb detection limit. According to AF1’s optical characteristics, a strong broad absorption band with a center wavelength of 385 nm in THF may be seen. This band is predominantly dominated by the coumarin chromophore. Due to effective quenching by the thiazoline N lone pair through a photoinduced electron transfer process, the resulting fluorescence emission maximum at 496 nm exhibits an incredibly low quantum yield (f) of 0.004. The nonconjugated AF1 is basically non-fluorescent. When As3+ is added (as AsI3, but AsCl3 also produces comparable effects), AF1’s fluorescence intensity increases by around 25 times. This strong turn-ON response is accompanied by red shifts in the absorbance maxima from 385 to 464 nm, which are indicative of benzothiazole C6-CF3 production. The commercially available coumarin-6 (analogue of C6-CF3 with hydrogen replacing the CF3 group) and other coumarin-benzothiazole compounds typically exhibit fluorescence via an internal-charge-transfer (ICT) mechanism. As a result, at 298 K, AF1 functions as an efficient OFF–ON fluorescence sensor for As3+ in organic media. The sensing process probably entails bis-coordination of the Schiff-base thiolate form of AF1 to the thiolate anion, which then attacks the C-N carbon and loses a proton to produce the benzothiazole [58]. The structures of the chemosensors 19-21 and their interaction with Cr3+ ion are shown in Figure 6.
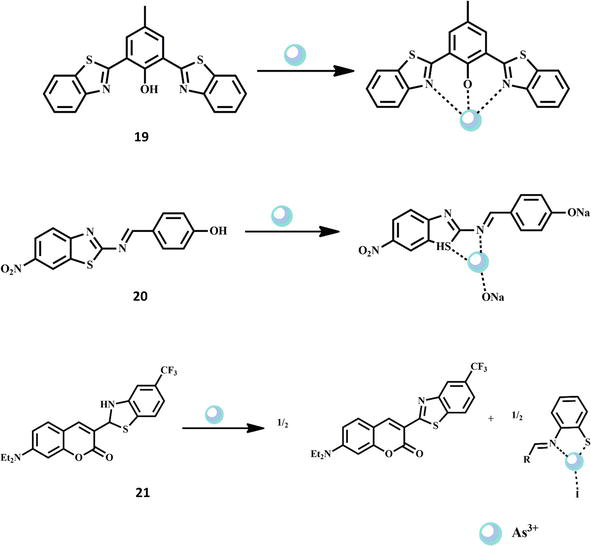
Figure 6.
Formation of As3+ complexes with Schiff base chemosensors 19–21.
6. Detection of other cations
A fluorogenic and chromogenic chemosensor 22 with great sensitivity was created. The developed chemosensor was distinguished by contemporary analytical methods. The completely characterized chemosensor was utilized to detect the presence of Au3+ ions in acetonitrile-containing aqueous media in the presence of a variety of competing analytes, including cations (chlorides salts of Au3+, Cr3+, Fe3+, Zn2+, Cd2+, Pb2+, Hg2+, Pt2+, Pd2+, perchlorates of Mn2+, Co2+, Ni2+, Cu2+, and nitrate salts of Ag+, and Al3+). The chemosensor 22 only demonstrated a selective response to Au3+ ions and revealed a 696-fold fluorescence increase toward Au3+. The structure of the chemosensor 22 and its interaction with Au3+ ion are shown in Figure 7. The limit of detection was determined to be 1.51 M. The chemosensor 22 also had a colorimetric response that ranged from colorless to pink. These imine bond (C- -N) hydrolysis-induced fluorometric and colorimetric responses were caused by Au3+ ions. Additionally, chemosensor 22 was successfully used to determine the presence of Au3+ ions in living MC3T3 cells [59]. For the purpose of detecting Y3+, a new tetraphenylethylene decorated with m-aminobenzoic acid (chemosensor 23) was designed and produced with an 89% yield. Based on the AIE effect, it displayed the far-red fluorescence at 550–670 nm in aqueous environments. It demonstrated strong Y3+ selectivity across all types of metal ions and a clear “turn-on” fluorescence, which was seen for the first time for a Y3+ fluorescence sensor. The detection threshold for Y3+ was as low as 2.23 × 10−7 M. Fluorescence titration helped to clarify the sensor mechanism for a 1:1 stoichiometric ratio. The selective sensing of Y3+ was also effectively used to identify Y3+ for tested paper, a simulated water sample, and bio-imaging of live cells, indicating the strong practical application potential of this fluorescent sensor on detecting Y3+ in diverse water media and living body environments [60]. Similarly, a new fluorescent chemosensor 24 was architected for the recognition of Y3+ ions. The probe 2-hydroxy-1-naphtaldehyde salicyloylhydrazone was described in detail. For the determination of yttrium in THF, a very sensitive fluorescent approach and the fluorescence method were developed based on the chelation reaction; they can detect traces of Y3+ with a naked eye in sunlight [61]. The structures of the chemosensors 23–24 and their interactions with Y3+ ion are shown in Figure 7. For the detection of Ga3+, a simple Schiff-base based on fluorene and salicylaldehyde was created. Using NMR titration, ESI-mass spectrometry analysis, photophysical experiments, and DFT simulations, the sensing behaviors of chemosensor 25 with Ga3+ were investigated. In the presence of a variety of cations, including K+, Cu2+, Co2+, Ca2+, Fe3+, Cr3+, Ag+, Na+, Ni2+, In3+, Fe2+, Mg2+, Cd2+, Hg2+, Mn2+, Pb2+, and Al3+, the chemosensor 25 produced a highly stable complex and showed good selectivity for Ga3+. In particular, the detection threshold for Ga3+ on chemosensor 25 was as low as 10 nM [62]. Chemosensor 26 and 27 were recently used for the effective detection of Ga3+ ions. The symmetrical Schiff base chemosensor 26 (N′2,N′5-bis((E)-2-hydroxybenzylidene)thiophene-2,5-dicarbohydrazide) and chemosensor 27 (N′5,N′7-bis((E)-2-hydroxybenzylidene)-2,3-dihydrothieno[3,4-b]) are both produced from thiophene. Fluorescence turn-on behavior was observed for Ga3+ detection. After chelation with Ga3+, both of them demonstrated considerable fluorescence amplification in MeCN/H2O buffer solution. The sensors, on the other hand, displayed ultralow detection limits of 3.90 × 10−9 M and 3.97 × 10−9 M, respectively. A 1:2 stoichiometry between the sensors and the Ga3+ was seen in the Job’s plots, with association constants of 3.89 × 109 M2 and 8.59 × 109 M2, respectively. Theoretical investigations into molecule configuration, charge distribution, molecular orbitals, electronic transitions, and energy change results were produced in order to further explain the differences in sensing performance between the two sensors [63]. The structures of the chemosensors 25–27 and their interactions with Ga3+ ion are shown in Figure 8.
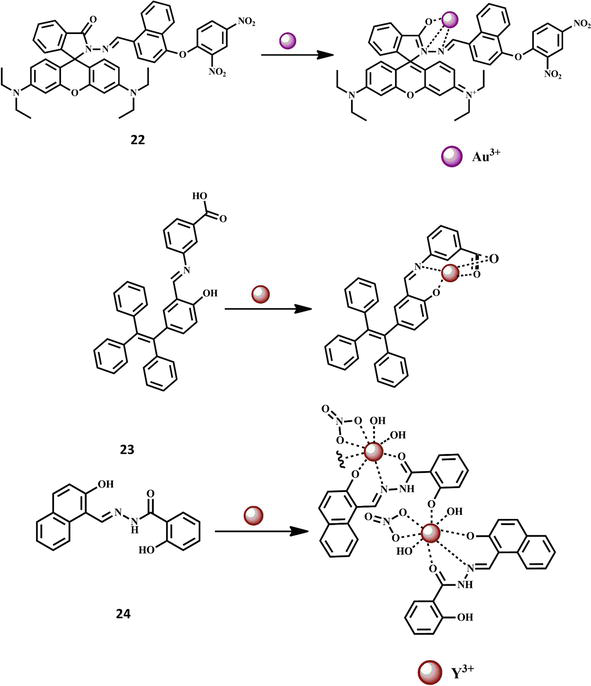
Figure 7.
Formation of Au3+ and Y3+ complexes with Schiff base chemosensors 22 and 23–24 respectively.
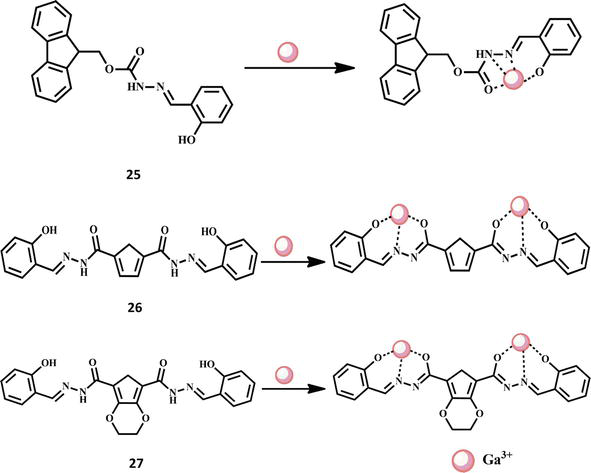
Figure 8.
Formation of Ga3+ complexes with Schiff base chemosensors 25–27.
7. Performance evaluation
We reviewed spectrofluorometric technique in this chapter for the identification and quantification of various cations in various environmental, biological, and agricultural materials. With a very low detection limit, many of these chemosensors have demonstrated remarkable sensitivity and selectivity for certain heavy metal analytes. The effectiveness of several Schiff base-based chemosensors for the detection of various cations is discussed. The best performing Schiff bases for sensing various cations were suggested after comparing the detection limit values. For the targeted detection of mercury ions, the chemosensors 1–6 were employed. With a detection limit of 8.3 nM, chemosensor 3 was discovered to be the most sensitive of these chemosensors. Cd2+ was detected using chemosensors 7–11. Fluorescence spectroscopy demonstrated the outstanding performance of chemosensor 7 for Cd2+. The calculated detection threshold is 14.8 nM. Additionally, the use of chemosensor 7 for the detection of Cd2+ in real water samples was successful. Similar to this, when compared to chemosensors 12–16, chemosensor 13 had the best performance for Cr3+ detection. With a low detection limit of 0.94 nM, the chemosensor responded to Cr3+ by turning on its fluorescence. For the detection of Pd2+, two different chemosensors using fluorescence (17–18) were employed. One of these chemosensors, 18 (with an outstanding detection limit of 0.0188 μM), was discovered to be very effective and sensitive for Pd2+. Compared to chemosensors 19, 20, and 21 for the detection of As3+, turn-on chemosensor 20 showed great sensitivity with a detection limit of 7.2 ppb in the aqueous medium. The Schiff base fluorescent chemosensor 22 displayed high sensitivity toward Au3+ with a detection limit of 1.51 μM, while the chemosensor 24 displayed good sensitivity toward Y3+ with a detection limit of 0.013 ppb. These findings come from the discussion on the sensing of Au3+, Y3+, and Ga3+. A detection limit of 3.9 nm for the chemosensor 26 indicated that it had good sensitivity to Ga3+ [64]. The different chemosensors and its analytical parameters for the chemosensing analysis of heavy metals are listed in the Table 1.
8. Conclusion
Schiff base compounds are easily made in laboratories using various reaction procedures. With the help of various analytical techniques, Schiff bases have been used to detect a variety of metal ions, such as Hg2+, Cd2+, Cr3+, Pd2+, As3+, Au3+, Y3+, and Ga3+. This chapter has discussed how various Schiff base probes can be used as chemosensors to detect metal ions (cations). A stable complex can be formed when a metal center coordinates with the nitrogen atom of azomethine, which is a possible ligating site. In terms of metal cation detection, excellent results have been reported. However, the development of chemosensors with high selectivity and sensitivity for harmful metal cations that can operate in a variety of media is crucial. The availability, biological compatibility, and structural flexibility of Schiff base derivatives are further factors that support research.
References
- 1.
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management. 2011; 92 :407-418. DOI: 10.1016/j. jenvman.2010.11.011 - 2.
Li M, Gou H, Al-Ogaidi I, Wu N. Nanostructured sensors for detection of heavy metals: A review. ACS Sustainable Chemistry & Engineering. 2013; 1 :713-723. DOI: 10.1021/sc400019a - 3.
Sharma N, Sodhi KK, Kumar M, Singh DK. Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environmental Nanotechnology, Monitoring & Management. 2021; 15 :100388. DOI: 10.1016/j.enmm.2020.100388 - 4.
Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020; 6 :04691. DOI: 10.1016/j.heliyon.2020.e04691 - 5.
Lentini P, Zanoli L, Granata A, Signorelli SS, Castellino P, Dell’Aquila R. Kidney and heavy metals - The role of environmental exposure (review). Molecular Medicine Reports. 2017; 15 :3413-3419. DOI: 10.3892/mmr.2017.6389 - 6.
Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicology Mechanisms and Methods. 2020; 30 :167-176. DOI: 10.1080/15376516.2019.1701594 - 7.
Muro-Gonzalez DA, Mussali-Galante P, Valencia-Cuevas L, Flores-Trujillo K, Tovar-Sanchez E. Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environmental Science and Pollution Research. 2020; 27 :40187-40204. DOI: 10.1007/s11356-020-10026-5 - 8.
Anyanwu BO, Orisakwe OE. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. Journal of Environmental Science and Health, Part C: Environmental Carcinogenesis and Ecotoxicology Reviews. 2020; 38 :204-244. DOI: 10.1080/26896583.2020.1782116 - 9.
Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. Journal of Molecular Liquids. 2019; 290 :111197. DOI: 10.1016/j.molliq.2019.111197 - 10.
Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: A review of toxic actions and effects. Interdisciplinary Toxicology. 2020; 12 :45-70. DOI: 10.2478/intox-2019-0007 - 11.
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environmental Science and Pollution Research. 2016; 23 :8244-8259. DOI: 10.1007/s11356-016-6333-x - 12.
Zhou Q , Lei M, Liu Y, Wu Y, Yuan Y. Simultaneous determination of cadmium, lead and mercury ions at trace level by magnetic solid phase extraction with Fe@ Ag@Dimercaptobenzene coupled to high performance liquid chromatography. Talanta. 2017; 175 :194-199. DOI: 10.1016/j.talanta.2017.07.043 - 13.
Bansod BK, Kumar T, Thakur R, Rana S, Singh I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosensors & Bioelectronics. 2017; 94 :443-455. DOI: 10.1016/j.bios.2017.03.031 - 14.
Zinoubi K, Majdoub H, Barhoumi H, Boufi S, Jaffrezic-Renault N. Determination of trace heavy metal ions by anodic stripping voltammetry using nanofibrillated cellulose modified electrode. Journal of Electroanalytical Chemistry. 2017; 799 :70-77. DOI: 10.1016/j.jelechem.2017.05.039 - 15.
Colim AN, do Nascimento PC, Wiethan BA, Adolfo FR, Dresch LC, de Carvalho LM, et al. Reversed-phase High-performance Liquid Chromatography for the Determination of 15 Rare Earth Elements in Surface Water Sample Collected in a Mining Area from Lavras do Sul/RS. Vol. 82. Brazil: Chromatographia; 2019. pp. 843-856. DOI: 10.1007/s10337-019-03709-w - 16.
Shih TT, Hsu IH, Chen SN, Chen PH, Deng MJ, Chen Y, et al. A dipole-assisted solid-phase extraction microchip combined with inductively coupled plasma-mass spectrometry for online determination of trace heavy metals in natural water. Analyst. 2015; 140 :600-608. DOI: 10.1039/c4an01421a - 17.
Comby S, Tuck SA, Truman LK, Kotova O, Gunnlaugsson T. New trick for an old ligand! The sensing of Zn(II) using a lanthanide based ternary Yb(III)-cyclen-8-hydroxyquinoline system as a dual emissive probe for displacement assay. Inorganic Chemistry. 2012; 51 :10158-10168. DOI: 10.1021/IC300697W - 18.
Aletti AB, Gillen DM, Gunnlaugsson T. Luminescent/colorimetric probes and (chemo-) sensors for detecting anions based on transition and lanthanide ion receptor/binding complexes. Coordination Chemistry Reviews. 2018; 354 :98-120. DOI: 10.1016/J.CCR.2017.06.020 - 19.
Gunnlaugsson T, Bichell B, Nolan C. A novel fluorescent photoinduced electron transfer (PET) sensor for lithium. Tetrahedron Letters. 2002; 43 :4989-4992. DOI: 10.1016/S0040-4039(02)00895-X - 20.
Di W, Sedgwick AC, Thorfinnur Gunnlaugsson EU, Akkaya JY, James TD. Fluorescent chemosensors: The past, present and future. Chemical Society Reviews. 2017; 46 :7105-7123. DOI: 10.1039/C7CS00240H - 21.
Tidwell TT. Hugo (Ugo) Schiff, Schiff bases, and a century of β-lactam synthesis. Angewandte Chemie, International Edition. 2008; 47 :1016-1020. DOI: 10.1002/anie.200702965 - 22.
De La Hoz A, Díaz-Ortiz A, Prieto P. R.S.C. Green Chem. In: Microwave-assisted Green Organic Synthesis. UK: Royal Society of Chemistry; 2016. pp. 1-33. DOI: 10.1039/9781782623632-00001 - 23.
Liu YT, Sheng J, Yin DW, Xin H, Yang XM, Qiao QY, et al. Ferrocenyl chalcone-based Schiff bases and their metal complexes: Highly efficient, solvent-free synthesis, characterization, biological research. Journal of Organometallic Chemistry. 2018; 856 :27-33. DOI: 10.1016/j.jorganchem.2017.12.022 - 24.
Naeimi H, Salimi F, Rabiei K. Mild and convenient one pot synthesis of Schiff bases in the presence of P2O5/Al2O3 as new catalyst under solvent-free conditions. Journal of Molecular Catalysis A: Chemical. 2006; 260 :100-104. DOI: 10.1016/j.molcata.2006.06.055 - 25.
Kwon MS, Kim S, Park S, Bosco W, Chidrala RK, Park J. One-pot synthesis of imines and secondary amines by Pd-catalyzed coupling of benzyl alcohols and primary amines. The Journal of Organic Chemistry. 2009; 74 :2877-2879. DOI: 10.1021/jo8026609 - 26.
Khan E, Khan S, Gul Z, Muhammad M. Medicinal importance, coordination hemistry with selected metals (Cu, Ag, Au) and chemosensing of thiourea derivatives. A review. Critical Reviews in Analytical Chemistry. 2020; 50 :1-23. DOI: 10.1080/10408347.2020.1777523 - 27.
Kaur B, Kaur N, Kumar S. Colorimetric metal ion sensors – A comprehensive review of the years 2011-2016. Coordination Chemistry Reviews. 2018; 358 :13-69. DOI: 10.1016/j.ccr.2017.12.00 - 28.
Gunnlaugsson T, Ali HDP, Glynn M, Kruger PE, Hussey GM, Pfeffer FM, et al. Journal of Fluorescence. 2005; 15 :287. DOI: 10.1007/s10895-005-2627-y - 29.
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, et al. Chemical Reviews. 1997; 97 :1515-1566. DOI: 10.1021/cr960386p - 30.
Rotkiewicz K, Grellmann KH, Grabowski ZR. Reinterpretation of the anomalous fluorescense of p-n,n-dimethylamino-benzonitrile. Chemical Physics Letters. 1973; 19 :315-318. DOI: 10.1016/0009-2614(73)80367-7 - 31.
Rettig W. Photophysical and photochemical switches based on twisted intramolecular charge transfer (TICT) states. Applied Physics B Photophysics and Laser Chemistry. 1988; 45 :145-149. DOI: 10.1007/BF00695283 - 32.
Ito A, Ishizaka S, Kitamura N. A ratiometric TICT-type dual fluorescent sensor for an amino acid. Physical Chemistry Chemical Physics. 2010; 12 :6641-6649. DOI: 10.1039/B924176K - 33.
Rettig W, Lapouyade R. Fluorescence probes based on twisted intramolecular charge transfer (TICT) states and other adiabatic photoreactions. In: Lakowicz JR, editor. Topics in Fluorescence Spectroscopy, Probe Design and Chemical Sensing. Vol. 4. New York: Plenum Press; 1994. p. 109. DOI: 10.1007/0-306-47060-8_5 - 34.
Lodeiro C, Pina F. Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coordination Chemistry Reviews. 2009; 253 :1353. DOI: 10.1016/j.ccr.2008.09.008 - 35.
Wu JS, Zhou JH, Wang PF, Zhang XH, Wu SK. New fluorescent chemosensor based on exciplex signaling mechanism. Organic Letters. 2005; 7 :2133. DOI: 10.1021/ol0504648 - 36.
Chen WB, Elfeky SA, Nonne Y, Male L, Ahmed K, Amiable C, et al. A pyridinium cation–π interaction sensor for the fluorescent detection of alkyl halides. Chemical Communications. 2011; 47 :253. DOI: 10.1039/C0CC01420F - 37.
Shellaiah M, Rajan YC, Balu P, Murugan A. A Pyrene based schiff base probe for selective fluorescent turn-on detection of Hg 2+ ions with live cell application. New Journal of Chemistry. 2015; 39 :2523-2531. DOI: 10.1039/c4nj02367f - 38.
Wei T, Gao G, Qu W, Shi B, Lin Q , Yao H, et al. Selective fluorescent sensor for mercury(II) ion based on an easy to prepare double naphthalene Schiff base. Sensors and Actuators B: Chemical. 2014; 199 :142-147. DOI: 10.1016/j.snb.2014.03.084 - 39.
Muthusamy S, Rajalakshmi K, Zhu D, Zhu W, Wang S, Lee K-B, et al. Dual detection of mercury (II) and lead (II) ions using a facile coumarin-based fluorescent probe via excited state intramolecular proton transfer and photo-induced electron transfer processes. Sensors and Actuators B: Chemical. 2021; 346 :130534. DOI: 10.1016/j.snb.2021.130534 - 40.
İnal EK. A Fluorescent chemosensor based on schiff base for the determination of Zn2+, Cd2+ and Hg2+. Journal of Fluorescence. 2020; 30 :891-900. DOI: 10.1007/s10895-020-02563-6 - 41.
Su Q , Niu Q , Sun T, Li T. A simple fluorescence turn-on chemosensor based on Schiff-base for Hg2+−selective detection. Tetrahedron Letters. 2016; 57 (38):4297-4301. DOI: 10.1016/j.tetlet.2016.08.031 - 42.
Wang XM, Yan H, Chen Y, Bao HB. A new Schiff’s base fluorescent sensor for the selective detection of mercury ion. Advanced Materials Research. 2011; 239-242 :1105-1108. DOI: 10.4028/www.scientific.net/AMR.239-242.1105 - 43.
Mohanasundaram D, Bhaskar R, Gangatharan Vinoth Kumar G, Rajesh J, Rajagopal G. A quinoline based Schiff base as a turn-on fluorescence chemosensor for selective and robust detection of Cd2+ ion in semi-aqueous medium. Microchemical Journal. 2021; 164 :106030. DOI: 10.1016/j.microc.2021.106030 - 44.
Ma J, Dong Y, Zhou Y. Yan Wub and Zhen Zhao A pyridine based Schiff base as a selective and sensitive fluorescent probe for cadmium ions with “turn-on” fluorescence responses. New Journal of Chemistry. 2022; 46 :3348. DOI: 10.1039/D1NJ05919J - 45.
Maniyazagan M, Mariadasse R, Jeyakanthan J, Lokanath NK, Naveen S, Premkumar K, et al. Rhodamine based “turn–on” molecular switch FRET–sensor for cadmium and sulfide ions and live cell imaging study. Sensors and Actuators B: Chemical. 2017; 238 :565-577. DOI: 10.1016/j.snb.2016.07.102 - 46.
Aydin Z. A turn-on fluorescent sensor for cadmium ion detection in aqueous solutions. JOTCSA. 2020; 7 (1):277-286. DOI: 10.18596/jotcsa.638912 - 47.
Kolcu F, Erdener D, Kaya İ. A Schiff base based on triphenylamine and thiophene moieties as a fluorescent sensor for Cr (III) ions: Synthesis, characterization and fluorescent applications. Inorganica Chimica Acta. 2020:119676. DOI: 10.1016/j.ica.2020.119676 - 48.
Ding Y, Zhao C. A highly selective fluorescent sensor for detection of trivalent metal ions based on a simple schiff-base. Química Nova. 2018. DOI: 10.21577/0100-4042.20170229 - 49.
Chalmardi GB, Tajbakhsh M, Hasani N, Bekhradnia A. A new Schiff-base as fluorescent chemosensor for selective detection of Cr 3+: An experimental and theoretical study. Tetrahedron. 2018; 74 (18):2251-2260. DOI: 10.1016/j.tet.2018.03.046 - 50.
Khan S, Muhammad M, Algethami JS, Al-Saidi HM, Almahri A, Hassanian AA. Synthesis, characterization and applications of schiff base chemosensor for determination of Cr(III) Ions. Journal of Fluorescence. 2022; 32 (5):1889-1898. DOI: 10.1007/s10895-022-02990-7 - 51.
Abbasi A, Shakir M. An inner filter effect based Schiff base chemosensor for recognition of Cr(vi) and ascorbic acid in water matrices. New Journal of Chemistry. 2018; 42 (1):293-300. DOI: 10.1039/c7nj03677a - 52.
Bhanja A, Mishra S, Saha K, Sinha C. A fluorescence ‘turn-on’chemodosimeter for the specific detection of Pd 2+ by a rhodamine appended Schiff base and its application in live cell imaging. Dalton Transactions. 2017; 46 :9245-9252. DOI: 10.1039/C7DT01288H - 53.
Adak AK, Purkait R, Manna SK, Ghosh BC, Pathak S, Sinha C. Fluorescence sensing and intracellular imaging of Pd2+ ions by a novel coumarinyl-rhodamine Schiff base. New Journal of Chemistry. 2019; 43 :3899-3906. DOI: 10.1039/c8nj06511j - 54.
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. Journal of Toxicology. 2011; 2011 :1-13. DOI: 10.1155/2011/431287 - 55.
Yu H-S, Liao W-T, Chai C-Y. Arsenic carcinogenesis in the skin. Journal of Biomedical Science. 2006; 13 (5):657-666. DOI: 10.1007/s11373-006-9092-8 - 56.
Hong Y-S, Song K-H, Chung J-Y. Health effects of chronic arsenic exposure. Journal of Preventive Medicine and Public Health. 2014; 47 (5):245-252. DOI: 10.3961/jpmph.14.035 - 57.
Hu S, Yan G, Wu C, He S. An ethanol vapor sensor based on a microfiber with a quantum-dot gel coating. Sensors. 2019; 19 (2):300. DOI: 10.3390/s19020300 - 58.
Shah AQ , Kazi TG, Baig JA, Arain MB, Afridi HI, Kandhro GA, et al. Determination of inorganic arsenic species (As3+ and As5+) in muscle tissues of fish species by electrothermal atomic absorption spectrometry (ETAAS). Food Chemistry. 2010; 119 (2):840-844. DOI: 10.1016/j.foodchem.2009.08.0 - 59.
Paul S, Bhuyan S, Mukhopadhyay SK, Murmu NC, Banerjee P. Sensitive and selective in vitro recognition of biologically toxic As(III) by rhodamine based chemoreceptor. ACS Sustainable Chemistry & Engineering. 2019. DOI: 10.1021/acssuschemeng.9b00935 - 60.
Memon S, Bhatti AA, Bhatti AA, Ocak Ü, Ocak M. A new calix[4]arene Schiff base sensor for Hg2+ and Au3+. Journal of the Iranian Chemical Society. 2016; 13 (12):2275-2282. DOI: 10.1007/s13738-016-0946-3 - 61.
Jiang S, Chen S, Guo H, Yang F. “Turn-on” far-red fluorescence sensor for Y3+ based on Schiff-based tetraphenylethylene. Dyes and Pigments. 2020; 183 :108717. DOI: 10.1016/j.dyepig.2020.108717 - 62.
Zhang D, Zang Z, Zhou X, Zhou Y, Tang X, Wei R, et al. A selective fluorescence probe for yttrium(III) based on acylhydrazone Schiff base. Inorganic Chemistry Communications. 2009; 12 (11):1154-1156. DOI: 10.1016/j.inoche.2009.08.007 - 63.
Seong YL, Kwon HB, Tae GJ, So YK, Cheal K. A simple Schiff-base fluorescence probe for the simultaneous detection of Ga3+ and Zn2+. Inorganica Chimica Acta. 2017; 461 :127-135. DOI: 10.1016/j.ica.2017.02.019 - 64.
Xing Y, Liu Z, Li B, Li L, Yang X, Zhang G. The contrastive study of two thiophene-derived symmetrical Schiff bases as fluorescence sensors for Ga3+ detection. Sensors and Actuators B: Chemical. 2021; 347 :130497. DOI: 10.1016/j.snb.2021.130497