Abstract
Malaria is an acute febrile illness that is caused by infection with Plasmodium spp. parasites. Malaria is a serious illness and sometimes it may be fatal resulting in mortality and morbidity. The clinical picture painted in patients with malarial infection occurs following the release of the merozoites into the bloodstream following the rupture of infected red cells. In the infection with the P. falciparum, the commonest form affecting humans, all stages of red cells are infected making the infection quite severe as compared to infection with other species which infects the old and young red cells only which contributes to a small percentage of red cells. In this chapter, the Authors review the current knowledge about Malaria epidemiology, pathogenesis and anatomic pathology. The diverse clinical pictures as well as the association with genetic conditions and diseases are discussed.
Keywords
- malaria
- plasmodium malariae
- pathology
- histology
- gross and microscopic pathology
- cerabral malaria
- pathogenesis
1. Introduction
Malaria is an acute febrile illness that is caused by infection with
There were an estimated 241 million malarial cases in 2020 higher than malarial cases in 2019 (227 million cases), a difference of 14 million cases. The rise in malarial cases is thought to be associated with the COVID-19 pandemic, that led to service disruption. The case incidence of malaria from 2000 has declined from 81 per 1000 populations at risk to 56 per 1000 populations at risk. However, in 2020 the case incidence has increased to 59 per 1000 populations at risk. Globally, death due to malaria in 2020 is reported at 627000 which is a rise as compared to the trends in malaria death that has a seen a drop from 896,000 cases in 2000 to 558,000 cases in 2019. It is estimated that 68% (47,000 cases) of the additional deaths due to Malaria are attributed to disruption of services following COVID-19 pandemic. Globally, the malaria mortality rate as of 2020 is 15 per 100,000 population at risk which is higher compared to 2019 which was 14 per 100,000 population at risk. Among children under 5 years, the mortality rate in 2020 increased to 77% as compared to 76% in 2019. Globally, 96% of the malaria cases and deaths were from 29 out of 85 countries with malaria-endemic. Nigeria (26.8%), the Democratic Republic of Congo (12.0%), Uganda (5.4%), Mozambique (4.2%), Angola (3.4%), and Burkina Faso (3.4%) account for 55% of the cases globally. Half of the mortality cases were from Nigeria (31.9%), the Democratic Republic of Congo (13.2%), The United Republic of Tanzania (4.1%), and Mozambique (3.8%). The WHO African region accounts for 95% of the malarial cases and 96% of the malarial deaths. In the African region, 80% of the cases are among children under 5 years. In Africa, the number of malaria cases in 2020 is 233 per 1000 populations at risk higher than in 2019 at cases per 1000 population cases. The South-east Asia region accounts for 2% of the malaria cases globally among the nine malaria-endemic countries in 2020. India accounts for 83% of malaria cases due to infection by
There are attempts globally to eliminate malaria in different countries. According to WHO a country is declared malaria-free following at least 3 consecutive years with zero indigenous cases. Islamic Republic of Iran and Malaysia reported zero indigenous malaria cases for the third consecutive year and while Belize and Cabo Verde for the second consecutive time. China and El Salvador were certified malaria-free in 2021 following 4 years of zero malaria cases [1].
1.1 Plasmodium
Plasmodium was first described in the late nineteenth century by Charles Laveran and over time, many species have been discovered. Plasmodium is a unicellular eukaryote that cannot survive outside the host (obligate parasite). The parasite affects different hosts such as reptiles, birds, and mammals and it requires an insect host of the genera Culex and Anopheles. In humans
1.2 Lifecycle of palsmodium
The life cycle of plasmodium involves distinct stages in the mosquito and vertebrate host. The life cycle can be divided into the sexual phase that occurs in the insect and the asexual phase while inside the vertebrate host.
Upon taking the blood meal, the female anopheles mosquito releases the sporozoites into the bloodstream of an individual. The sporozoites enter the circulation and they attach and enter the hepatocytes through receptors for serum proteins thrombospondin and properdin. In the hepatocytes, sporozoites mature into schizonts. Schizonts are the multinucleated staged cells that form during asexual reproduction. The schizonts in the hepatocytes mature and enlarge which causes the hepatocytes to rupture and release thousands of merozoites into the circulation. For
2. Malaria pathogenesis and pathophysiology
The clinical picture painted in patients with malarial infection occurs following the release of the merozoites into the bloodstream following the rupture of infected red cells. The malaria parasite has a wide variety of symptoms which ranges from absent or very mild symptoms to severe cases and even death. Owing to this the infection is categorized into uncomplicated malaria and severe malaria. The incubation period varies depending on the individual factors and the species of plasmodium. The incubation period is the period of the introduction of the sporozoites to the development of the first symptoms. The incubation period varies from 7 to 30 days. However,
The release of the merozoites from the rupture of the liver cells infects the red cells. In the infection with the
The infected red cells appear sticky and adhered to the blood vessels causing obstruction of the blood flow and causing ischemia in the affected areas and organs. The sticky red cells as well clump together forming a rosette that if large enough have the potential to obstruct blood flow in major blood vessels. In addition, several proteins such as PfEMP1 contribute to the attachment of red cells on the endothelium leading to sequestration. The PfEMP1 bind to the endothelial cells through CD36, thrombospondin, VCAM1, ICAM1, and E-selectin. The sequestration of the red cells in the blood vessel endothelium and the ability of the body to take away damaged red cells mostly occur in the spleen contributing to splenomegaly in some patients. Sequestration of the red cells, and destruction of the red cells leading to anemia coupled with obstruction of blood flow results in the reduction of tissue perfusion causing fatigue, general body weakness, and ischemia.
2.1 P. falciparum
2.2 P. vivax
2.3 Plasmodium ovale
2.4 Plasmodium malariae
2.5 P. knowlesi
The infection of
3. Genetic factors and malaria
Genetic factors play a crucial role in influencing malaria infection. Two biological characteristics identified in protecting the certain type of malaria are sickle cell trait and negative for Duffy blood group [2].
Individuals with sickle cell traits are relatively protected from infection by
People who are negative for the Duffy blood group have shown to be resistant to infection with
Blood cell dyscrasias such as hemoglobin
4. Acquired immunity against malaria infection
Acquired immunity influences malaria infection in individuals and communities. Through repeated infection, an individual develops partially protective immunity. Despite the acquired immunity, the individuals may get infected by the malaria parasite but the severity of the illness will be less ad they may lack the typical symptoms. The acquired immunity allows the individual to mount the immune response to the presence of the parasite in the circulation leading to a reduction in the severity of the infection. The areas that have a high malarial infection such as
5. Sickle cell disease and malaria
Despite the presence of sickle cell trait conferring protestation against malarial infection over the healthy population, the presence of sickle cell disease does not confer any protection. Sickle cell disease occurs when the individual has a mutation in both alleles resulting in homozygous HbS. Sickle cell disease carries the worst prognosis with infection by the malarial parasite, especially
6. Clinical features of cerebral malaria
Cerebral malaria is the most severe neurological complication that arises following infection with
The development of cerebral malaria following infection with
Cerebral malaria is fatal and without treatment, the mortality rates increase exponentially. In children treated with intravenous antimalarial medication, the mortality is about 5–20%. However, despite the recovery, many children sustain significant brain injuries. About 11% have gross neurological deficits which may improve with time while 25% have long-term impairments, especially in cognition, motor, and behavior domain. About 10% develops epilepsy. The risk factors include seizures, deep and prolonged coma, intracranial hypertension, and hypoglycemia.
7. Congenital malaria
Congenital malaria refers to the infection with malaria parasite present in the peripheral smear of the newborn from 24 hours to 7 days of life. It is thought that the parasite can infect the placenta and gain access to fetal circulation. Congenital malaria is rare but it is fatal if not detected earlier in newborns. However, in areas where malaria is endemic, congenital malaria is rare as compared to other areas. This is because, in malaria-endemic areas, the maternal have had repeated attacks thus developing acquired immunity. The antibodies in the maternal circulation are therefore protected against the development of congenital malaria and protection in the early stages of life. The occurrence of congenital malaria is at 0.3% in the immune mothers and 7.4% in the non-immune mothers. The symptoms of congenital malaria occur within 10 to 30 days of life. They include fever, anemia, and splenomegaly in most cases. Other presentations may include hepatomegaly, jaundice, loose stool, and poor feeding among others. Since this presentation mimics most conditions in the neonates such as neonatal jaundice and neonatal sepsis, the diagnosis is often missed leading to mortality and morbidity [5, 6].
Congenital malaria is of concern among neonates. Given that the parasite is present in utero, the human body learns to recognize the parasite as self, contrary to the normal case where the parasite is considered an antigen to cause activation of immune responses. The parasite, therefore, can multiply and cause damage to red cells without evoking immune responses as in the case of a healthy individual. Therefore, the newborn experience excessive hemolysis and resultant organomegaly. Lack of the ability to recognize the parasite as an antigen coupled with an immature immune system allows the parasite to multiply necessitating immediate treatment to improve the outcome among the neonates. Owing to intravascular hemolysis, the neonates present with anemia and splenomegaly which are the most common forms of presentation. Intravenous antimalarial medication is essential in management [5, 6].
8. Gross and microscopic pathology
8.1 Bone marrow
8.2 Spleen
Splenic enlargement is one of the findings of all types of malaria. However, with
Tropic splenomegaly syndrome is characterized by marked enlargement of the spleen with a spleen weighing 2–4.4 kgs. Usually seen among the adult population in Africa and India, presenting with a huge spleen. The spleen demonstrates marked hyperplasia of lymphoid with dilated sinuses. With splenomegaly, there is increased phagocytosis of red and white cells, and the patient has a picture of anemia, leucopenia and thrombocytopenia, though the general health is well maintained. There may be concomitant enlargement of the liver with lymphoreticular infiltration of sinusoids. The patients have high levels of IgG and M against malaria. However, the use of antimalarial medication reduces the splenic size [7, 8].
Treatment of malarial infection results in splenic regression, usually within weeks, but the duration may be protracted with large fibrotic spleen secondary to repeated malaria, though complete involution is common. The patient undergoing splenectomy has a risk of latent infection reactivation since the spleen plays a crucial role in the immune response against malaria.
8.3 Liver
In malarial infection, hepatomegaly also occurs in the early stages. The liver is enlarged, firm and tender. It may appear brown, gray or black following malaria pigment deposition. Microscopically, there is oedema and dilatation of hepatic sinusoids containing hypertrophied Kupffer cells and parasitized red cells. In severe malarial cases, shock and disseminated intravascular coagulation result in small areas of centrilobular necrosis. Prolonged infection results in stromal induration and diffuse proliferation of fibrous connective tissue, but changes of cirrhosis are absent [7, 8].
In repeated infection, there is significant hepatomegaly with associated splenomegaly, but no functional abnormality exists. Despite prolonged malarial infection having diffuse fibrous connective tissue proliferation, malaria is not a proven cause of liver cirrhosis.
8.4 Lungs
Acute pulmonary oedema may occur in a patient with
8.5 Cardiovascular system
Cardiovascular functioning is deranged with malarial infection, especially during the paroxysmal episode. There is peripheral vasodilation causing decreased blood pressure and postural hypotension, tachycardia, transient systolic murmur, muffled heart sound and occasional cardiac dilatation. In the patient with pre-existing cardiac dysfunction, malarial infection aggravates the dysfunction leading to fatal cardiac failure. The microcirculation changes involve myocardial capillaries congestion with lymphocytes, plasma cells and parasitized red cells. Pigment-laden macrophages are also seen microscopically [7, 8].
8.6 Gastrointestinal system
Malaria may manifest with abdominal symptoms such as nausea, vomiting, anorexia, abdominal distension and epigastric pain in the acute phase. Nausea and vomiting are usually central in origin. In addition, the patient may have watery diarrhea, mimicking gastroenteritis or cholera. Some patients may experience severe abdominal colic mimicking appendicitis and acute abdomen.
8.7 Kidneys
Kidneys may be affected during malarial infection. Acute diffuse malarial nephritis rarely occurs with patients exhibiting hypertension, oedema and albuminuria. However, albuminuria alone is common during an acute attack.
Severe disease with
8.8 Central nervous system
As mentioned, the CNS presentation is due to the dissemination of malarial parasites into the brain, paroxysmal fever and side effects of antimalarial drugs. The patient presents with headache, vomiting, delirium, anxiety and restlessness during fever paroxysms, with symptoms resolving once the fever normalizes. Chloroquine, quinine, mefloquine and halofantrine can lead to vertigo, restlessness, hallucinations, confusion, delirium, tinnitus, dizziness, convulsions and sometimes frank psychosis. Quinine is associated with hypoglycemic coma, while artemisinin cause brainstem dysfunction based on animal studies [7, 9].
Macroscopically, the brain is usually edematous during the autopsy, appearing leaden or plum colored with a cut surface slatey gray hue. The sulci are narrowed, and the gyri flattened due to brain swelling. The small blood vessels are congested with parasitized red cells. Mature forms of parasites including schizonts are found in brain biopsies. The large vessels demonstrate evidence of margination, where the parasites are arranged in a layer along the endothelium. Despite this, the endothelium also shows pseudopodial projections, which may be in close apposition to the knobs on the surface of parasitized red cells. There is numerous petechial hemorrhage proximal to the occlusive plug of end arterioles in the white matter. The ring hemorrhage is diffuse in the brain with hemorrhage containing fibrin, pigmented parasites, free pigments and admixed fibrin. The uninfected red cells are seen in surrounding hemorrhage. Diirck’s granulomata may be seen in areas of hemorrhage characterized by a small collection of microglial cells surrounding an area of demyelination. In addition, the brain demonstrates the abrupt transition from gray to white matter which, together with ring hemorrhage, are classical findings of cerebral malaria (Figures 1-3) [9].
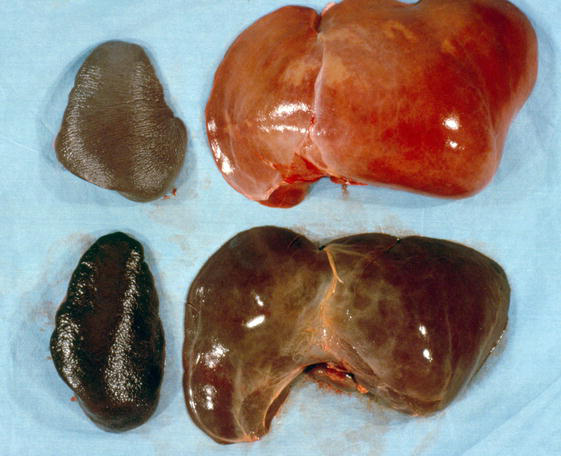
Figure 1.
Liver and Spleen Malaria. Gross pathology of the liver and spleen At the top of this picture are the spleen (left) and liver (right) from an autopsy of a child. They show a normal spleen and liver appearance. At the bottom of the picture are the spleen and liver from the autopsy of a child who died of malaria, which are a darker color than the normal organs. The dark color comes from extreme congestion and heavy deposition of haemozoin. (From:
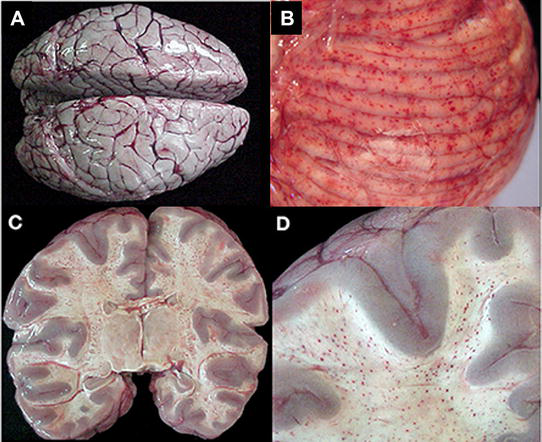
Figure 2.
Gross pathology of cerebral malaria. The vast majority of cases, regardless of diagnosis, showed brain swelling with flattened gyri and narrowed sulci (A). In this example, the brain has the classic “slate gray” to “purple” appearance of CM which is possibly due to malaria pigment within vessels (A). The cerebellum had petechial hemorrhages in both the gray and white matter and thus, visible on the surface grossly (B). In the classic CM2 appearance, petechial hemorrhages are seen diffusely in the white matter throughout the brain (C). A higher magnification demonstrates the abrupt transition from white to gray matter and the lack of hemorrhages in the gray (D). From Milner et al. [
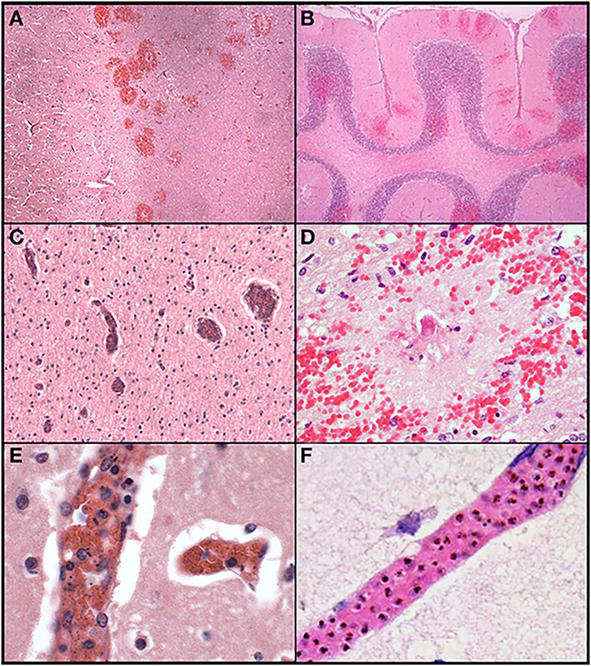
Figure 3.
Histological features of cerebral malaria. The abrupt transition from gray to white matter (A) and the presence of ring hemorrhages are demonstrated in this classic case of CM (CM2, 100X, H&E). The cerebellum (B) with ring hemorrhages in all levels including white and gray matter are shown (100x, H&E). Visibly congested blood vessels (C) even at low power may be the result of dense sequestration downstream; these vessels can contain both parasitized and uninfected red bloods (200X, H&E). The classic appearance of a ring hemorrhage with fibrin (D) is shown; these hemorrhages can also include pigmented parasites, free pigment, and admixed fibrin within the microvessel at the nexus of the lesion; uninfected erythrocytes constituting the surrounding hemorrhage are seen (400X, H&E). Two examples of sequestration showing predominantly early (less pigmented) parasites (E), and late stage (more pigmented) parasites (F) densely packing vessels (1000X, H&E). From Milner et al. [
References
- 1.
World Health Organization. World malaria report 2021. 2021 - 2.
Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002; 359 :1311-1312 - 3.
Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Laboratory Investigation; A Journal of Technical Methods and Pathology. 1991; 64 (5):648-655 - 4.
Rénia L, Howland SW, Claser C, Gruner AC, Suwanarusk R, Teo TH, et al. Cerebral malaria: Mysteries at the blood-brain barrier. Virulence. 2012; 3 (2):193-201 - 5.
Thapar RK, Saxena A, Devgan A. Congenital malaria. Medical Journal, Armed Forces India. 2008; 64 (2):185 - 6.
Lesko CR, Arguin PM, Newman RD. Congenital malaria in the United States: A review of cases from 1966 to 2005. Archives of Pediatrics & Adolescent Medicine. 2007; 161 (11):1062-1067 - 7.
Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. Dec 2004; 20 (12):597-603. doi: 10.1016/j.pt.2004.09.006. PMID: 15522670 - 8.
Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum -infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Reviews in Molecular Medicine. 2009;11 :e16. DOI: 10.1017/S1462399409001082 - 9.
Milner DA Jr, Whitten RO, Kamiza S, Carr R, Liomba G, Dzamalala C, et al. The systemic pathology of cerebral malaria in African children. Frontiers in Cellular and Infection Microbiology. 2014; 4 :104. DOI: 10.3389/fcimb.2014.00104